First law of thermodynamics and the ideal
gas law
Problem:
5 moles of gas in a cylinder undergo
an isobaric expansion starting at 293 K. The cylinder is initially 50 cm
tall. The radius of the cylinder is 10 cm and Δy is 1 cm. How much work is
done by the gas?
Solution:
- Concepts:
Isobaric expansion, the ideal gas law
- Reasoning:
The process is isobaric, the pressure P is
constant. If the volume increase from Vinitial to Vfinal,
then the work done by the gas is W = P(Vfinal - Vinitial)
- Details of the calculation:
W = PΔV =
(nRT/V)ΔV = (5*8.31*293/(0.5 π 0.12) )*(0.01 π 0.12)
J
= 243 J
Problem:
One mole of an ideal gas does 3000 J of work on its surroundings as it
expands isothermally to a final pressure of 1 atm and volume of 25 L. Determine
(a) the initial volume and
(b) the temperature of the gas.
Solution:
- Concepts:
Ideal gas law: PV = nRT, work done on the system: W = -∫PdV
Energy conservation: ΔU = ΔQ + ΔW
- Reasoning:
For an isothermal process W = nRT ln(Vi/Vf).
We are given n, Pfinal, and Vfinal, and we are told
that the temperature is constant.
- Details of the calculation:
(a) For an isothermal process the temperature is constant.
Therefore PV = nRT = constant.
PV = 101000 Pa*25*10-3 m3 = nRT
W = nRT ln(Vi/Vf) for an isothermal process.
W/(nRT) = -3000 J/(101000 Pa*25*10-3 m3) = -1.19
(The work done by the gas is -W.)
Vf/Vi = exp(1.19) = 3.28
Vi = (25*10-3 m3)/3.28 = 7.62*10-3
m3 = 7.62 L
(b) For an ideal gas PV = nRT. 101000 Pa*25*10-3 m3 =
(8.31 J/K)T.
T = 303.85 K.
Problem:
A cylinder contains He gas. In its initial state a the cylinder
Volume is Va = 4.23*10-3 m3 and the pressure is
Pa = 1.19*10-5 Pa. The volume is isothermically reduced
until the system reaches a state b with Vb = 0.581*10-3
m3. This process is followed by an isobaric expansion which allows
the system to reach a state c with Vc = Va. The
cycle finishes with an isochoric or isometric (constant volume) process that
brings the system back to state a.
(a) Draw the cycle in a P-V diagram.
(b) Find the work done by the system going from b to c.
(c) Find the work done by the system in one cycle.
(d) Find the fractional change of the temperature of the system going from b
to c.
(e) When the system goes from c to a, does it absorb or release
heat? Find the amount of heat absorbed or released.
(f) When the system goes from b to c, does it absorb or release
heat? Find the amount of heat absorbed or released.
Solution:
- Concepts:
Ideal gas law: PV = nRT, work done on the system: W = -∫PdV
Energy conservation: ΔU = ΔQ - ΔW
increase in internal energy of a system = heat put into the system - work
done by the system on its surroundings,
- Reasoning:
An isothermal process occurs at constant temperature. P = nRT/V . Since
the internal energy of a gas is only a function of its temperature, ΔU = 0
for an isothermal process. For the isothermal expansion of an ideal gas we
haveW = ∫V1V2PdV = ∫V1V2nRT
dV/V = nRT∫V1V2 dV/V = nRT ln(V2/V1).
W is negative if V2 < V1. Since ΔU = 0, the heat
transferred to the gas is ΔQ = W.
An isobaric is a process that occurs at constant pressure. We then have
W = P∫V1V2 dV = P(V2 - V1).
If the pressure of an ideal gas is kept constant, then the temperature must
increase as the gas expands. (PV/T = constant.) Heat must be added during
the expansion process.
An isometric process takes place at constant volume. Then W = 0 and ΔU = ΔQ.
All the heat added to the system goes into increasing its internal energy.
- Details of the calculation:
(a)

(b) Wbc = Pb(Va-Vb) = Pa(Va/Vb)
(Va-Vb) = 3.16*10-7 J,
U = (3/2)NkT = (3/2)nRT = (3/2)PV, ΔUbc = (3/2)Pb(Va - Vb)
= 4.74*10-7 J,
ΔQbc = ΔUbc + Wbc = 4.74*10-7 J + 3.16*10-7 J
= 7.9*10-7 J.
(c) Wab = nRT ln(Vb/Va) = PaVa
ln(Vb/Va) = -10-7 J. Wcycle = Wbc
+ Wab = 2.16*10-7 J.
(d) (Tc - Tb)/Tb = Pb(Va
- Vb)/nRTb = (Va - Vb)/Vb
= 6.28. The temperature increases by a factor of 6.2
(e) The system releases heat.
ΔWca = 0, ΔUca = ΔQca = (3/2)(Pa - Pb)Va
= -4.74*10-7 J.
The system releases heat.
(f) The system absorbs heat.
ΔQbc
= 7.9*10-7 J.
[ΔUab= 0.
ΔQab = Wab = nRT ln(Vb/Va) = -10-7
J.
ΔQcycle =
ΔQab +
ΔQbc + ΔQca = Wcycle = 2.16*10-7 J.]
Problem:
Air at 20.0 oC in the cylinder of a Diesel engine is compressed
from an initial pressure of 1.00 atm and volume of 800 cm3 to a
volume of 60 cm3. Assume that air behaves as an ideal gas with γ =
1.40 and that the compression is adiabatic. Find the final pressure and
temperature of the air. (Give numerical answers!)
Solution:
- Concepts:
Adiabatic processes
- Reasoning:
We are asked to assume that PVγ = constant, with γ = 1.40.
- Details of the calculation:
This is an adiabatic process where T and P both increase.
Using PiVi γ = PfVf
γ we have
Pf = Pi(Vi/Vf)γ
= (1 atm)(800 cm3/60 cm3)1.4 = 37.6 atm.
Since PV = nRT is valid for an ideal gas, and no gas escapes from the
cylinder we have
PiVi/Ti = PfVf/Tf
--> Tf = [PfVf/(PiVi)]Ti.
Tf = = (37.6 atm/ 1atm)(60 cm3/800 cm3)*293
K = 826 K = 553 oC.
Problem:
A quantity of an ideal monatomic gas consist of N atoms, initially at
temperature T1.
The pressure and volume are then slowly doubled, in such a way as to trace out a
straight line on the P-V diagram.
In terms of N, k, and T1, find
(a) the work done by the gas.
(b) If one defines an equivalent specific heat capacity (c = ΔQ/ΔT, where ΔQ is
the total heat transferred to the gas) for this particular process for the
above monatomic gas, what is its value?
Solution:
- Concepts:
The P-V diagram, the ideal gas law, the first law of thermodynamics
- Reasoning:
We are given P2 = 2 P1, V2 = 2 V1
and asked to find W and ΔQ.
- Details of the calculation:
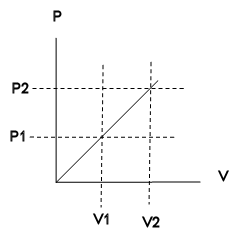
Given: P/V = P1/V1, P(V) = (P1/V1)V
Ideal gas law: PV = NkT, T2/T1 = P2V2/(P1V1)
= 4, T2 = 4 T1.
W = ∫V1V2PdV = (P1/V1) ∫V1V2VdV
= ½ (P1/V1)(V22 - V12)
= (3/2)P1V1 = (3/2)NkT1.
(b) ΔU = ΔQ - W, W = work done by the gas.
ΔQ = ΔU + W
U = (3/2)NkT, ΔU = (3/2)NkΔT = (3/2)Nk(T2 - T1)
= (3/2)Nk 3T1 = (9/2)NkT1.
ΔQ = (9/2)NkT1 + (3/2)NkT1 = 6NkT1.
c = ΔQ/ΔT = 6NkT1/(3T1) = 2Nk.
Problem:
Two cylinders A and B, with equal diameters have inside two
pistons with negligible mass connected by a rigid rod.
The pistons can move
freely.
The rod is a short tube with a valve. The valve is initially
closed.
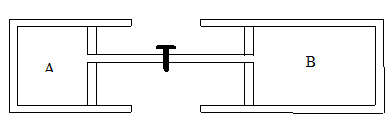
Cylinder A and its piston is adiabatically insulated and
cylinder B is in thermal contact with a thermostat which has the temperature T =
27o C.
Initially the piston of cylinder A is held fixed and
cylinder A contains m = 32 kg of argon at a pressure higher than the atmospheric
pressure. Cylinder B contains oxygen at atmospheric pressure (101.3 kPa).
Releasing the piston of cylinder A, it moves slowly (quasi-statically) until it
reaches equilibrium. At equilibrium the volume of the gas in cylinder A
has increased by a factor of eight, and in cylinder B the oxygen's density has
increased by a factor of 2. During this process the heat Q =
747.9*104 J is delivered to the thermostat.
(a) Use the ideal gas law and the first law of
thermodynamics to show that for the process taking place in cylinder A we have
TV(2/3) = constant.
(b) Calculate the parameters P, V, and T
of the argon in the initial and final states.
(c) After equilibrium has
been reached, the valve between the cylinders is opened. Calculate
the final pressure of the mixture of the gases.
The kilo-molar mass of argon
is 40 kg/kmol.
Solution:
- Concepts:
The ideal gas law, the first law of thermodynamics
- Reasoning:
We are asked to track P, V, and T of two quantities of
gas.
Given:
Ar: V2/V1 = 8, n = 800 mol.
O2: T1 = T2 = 300 K, ρ1/ρ2
= 2 --> P1/P2 = 2, since P = ρkT and T is constant.
- Details of the calculation:
(a) We consider argon an ideal
mono-atomic gas.
For an ideal gas PV = NkT.
For an adiabatic process
dU = -dW = -PdV.
But we also have U = N(3/2)kT for an ideal
gas. Therefore we have U = (3/2)PV,
dU = (3/2)(PdV + VdP).
Equating
our two expressions for dU we have -PdV = (3/2)(PdV + VdP),
dP/P + (5/3)dV/V
= 0. PV5/3 = constant, (PV)V(2/3)= constant,
TV(2/3)
= constant.
(b) The oxygen is in contact with a thermostat. Its
temperature and the internal energy are constant.
Since the net work
done by the gas in the system is zero, the change in the internal energy of
the argon is ΔU = Q.
We have for the argon ΔU = (3/2)nR(T2 - T1)
= (3/2)nRT1((V1/V2)(2/3) - 1) = Q.
T1 = (2/3)(1/n)(Q/R)(1/((V1/V2)(2/3)
- 1)) = (2/3)(1/800)(747.9*104/8.314)(4/3) K = 1000 K.
T2 = T¼ = 250K
For the
oxygen undergoing an isothermal process we have P1/P2
= ρ1/ρ2.
P1 = 1 atm, P2 = 2
atm = 2.026*105 N/m2.
From the equilibrium
condition we have that for the argon P2 = 2 atm = 2.026*105
N/m2.
For the argon P1 = P2(V2/V1)(T1/T2)
= 64 atm = 64.9*105 N/m2.
From the ideal gas law we
now have V1 = nRT1/P1 = 1.02 m3,
V2 = 8V1 = 8.16 m3.
So for the Ar gas:
P1 = 64.9*105
N/m2, V1 = 1.02 m3, T1 = 1000 K.
P2 = 2.026*105
N/m2, V2 = 8.16 m3, T2 = 250 K.
(c) When
the valve is opened the gases mix and in thermal equilibrium the final
pressure will be P and the temperature T = 27oC = 300K . The
total number of kilomoles n is constant. The total volume V of the
system is constant.
n = nAr + nO = PV/(RT)
Since the density of the oxygen increases by a factor of 2 during the
adiabatic expansion of the argon, we know that before the expansion the
volume of the oxygen was
VO2 i = 2*(VAr f - VAr i) = 14 VAr
i.
The total volume of gas therefore is V = 15 VAr i.
The number of moles of oxygen is nO = (1 atm)*(14 VAr i)/(R
300 K) = 580 mol
n = nAr + nO = (580 + 800) mol =
1380 mol
We now have P = nRT/V = 2.25*105 Pa = 2.2 atm.
Problem:
A container with helium is sealed by a movable heavy piston
with cross-sectional area A. Initially, the piston is in equilibrium, the
volume of the gas is V, and the system is in thermal equilibrium with its
surroundings. The piston is then slowly lifted by an external force a distance L
and held until the thermal equilibrium with the surroundings is re-established.
After that, the container is thermally insulated from the surroundings and
piston is released. Find the new equilibrium position of the piston with
respect to its initial position. Will the piston come to rest or will it
oscillate about this position? Neglect friction, the heat capacities of the
piston and the container, and the mass of the gas compared to the mass of the
piston.
Solution:
- Concepts:
The first law of thermodynamics, the ideal gas law
- Reasoning:
The system consists of a container filled with helium gas, which we treat as an
ideal, monatomic gas. The container is sealed by a moveable heavy piston. Let
us consider three separate states of initial, intermediate, and final.
State 1: Initial
 |
State 2: Intermediate
 |
State 3: Final
 |
For each
state we consider the implications of the ideal gas law, PV = nRT, and the first
law of thermodynamics,
dEint = dQ - dW, Eint = (3/2)nRT, dW = PdV.
- Details of the calculation:
(1)
The system is initially in equilibrium. We can apply the ideal gas law
to relate the pressure and volume of state 1 to temperature at state 1.
P1V = nRT1, T1 = P1V/(nR)
Environment: Penv + Mg/A = P1, where M is the mass of the
piston.
(2)
An external force raises the piston to a distance L. Heat is supplied by the
surrounding thermal reservoir to keep the temperature constant The change in
internal energy between state 1 and state 2 is zero.
ΔEint(1 --> 2) = 0. T2 = T1. P2 = nRT1/(V
+ AL). P2 < P1.
(3)
The system is now thermally insulated and the moveable piston is released. What
follows is an adiabatic process, ΔQ = 0.
The piston will accelerate. As it accelerates downward, work is done on the
gas, resulting in a change in its internal energy and temperature. The piston
will continue to accelerate downward until the pressure P3 = P1
= Penv + Mg/A.
Both the ideal gas law and the fact first law for
an adiabatic process yield an expression for T3/T1.
We equate these expressions to find the new equilibrium position.
The ideal gas law implies P1(V + Al) = nRT3, T3
= P1(V + Al)/(nR), where l is the distance of the new equilibrium
position above the initial position of the piston. T3/T1
= (V + Al)/V.
As the piston falls the first law of thermodynamics implies dEint = -PdV
= -PAdl'.
(3/2)nRdT = -PAdl' = -nRT Adl'/(V + Al').
dT/T = -(2/3)Adl'/(V + Al'),
ln(T3/T1) = (2/3)ln((V + AL)/(V + Al)),
T3/T1 = ((V + AL)/(V + Al))(2/3),
(V + Al)/V = ((V+AL)/(V + Al))(2/3),
(V + Al)5/3 = V*(V + AL)(2/3).
V + Al = V3/5*(V + AL)2/5.
Al = V3/5*(V + AL)2/5 - V.
If AL << V then Al = (2/5)AL, l = 2L/5.
The piston will
oscillate.
Problem:
One mole of a monatomic gas is
heated in such a way that its molar specific heat is 2R. During the heating,
the volume of the gas is doubled. By what factor does the temperature of the
gas change?
Solution:
- Concepts:
First law of thermodynamics
- Reasoning:
dU = dQ - PdV, where U is the internal energy of the system, P is the
pressure, V is the molar volume, and Q is the heat transferred to the gas by
the surroundings. For an ideal gas, U depends on the temperature only, U =
3RT/2.
Given dQ/dT = 2R,
dQ = 2RdT.
- Details of the calculation:
Using the ideal gas law, PV = RT, we have
dU = dQ - PdV = 2RdT - (RT/V)dV = (3R/2)dT
Therefore (RT/V)dV = (R/2)dT, or 2dV/V = dT/T, or V2/T = const.
Therefore, doubling the volume of the gas will increase its temperature by a
factor of four.
Problem:
One mole of an ideal gas undergoes transformations that take it from
point A to point B on a p-V diagram by two different paths α and β. Points
A and B have the same temperature T = T0 (this means that they
lie on an isotherm) and the pressure pA at point A is larger than
the pressure pB at point B. Path α takes the system from state A
to state I through an isochoric (constant volume) transformation, followed
by an isobaric (constant pressure) transformation from state I to state B. Path β takes the system from state A to state II through an isobaric
transformation, followed by an isochoric transformation from state II to
state B. Assume that pA and pB are known.
(a) Find VA and VB in terms of the known quantities pA,
pB and T0.
(b) In a p-V diagram plot path α and path β
(c) Assuming that the heat capacities CV and CP are
constants what is the heat flow into the gas for each path? Give your
results in terms of pA, pB, T0, CV
and Cp. In each case is heat absorbed or released by the system?
Why?
(d) In absolute value, for what process is the heat larger? Why?
Solution:
- Concepts:
The ideal gas law
- Reasoning:
We use the ideal gas law together with these definitions:
at constant pressure: dQ = nCPdT
at constant volume: dQ = nCVdT
- Details of the calculation:
(a) Ideal gas law: VA = RT0/pA,
VB = RT0/pB.
(b) Points A and B lie on an isotherm.
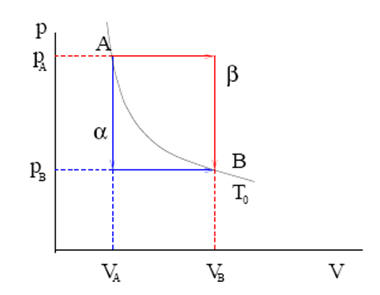
(c) For path α:
ΔQα
= ∫T0T1 CV dT = ∫T1T0
CP dT = CV(T1 - T0) +
Cp(T0 - T1) = (T0 - T1)(CP
- CV)
Here T1 is the temperature of the state with VA
and pB.
T1 = pBVA/R = pBT0/PA.
Therefore ΔQα
= T0(1 - pB/pA)(CP - CV).
For path β:
ΔQβ
= ∫T0T2 CP dT = ∫T2T0
CV dT = CP(T2 - T0) +
CV(T0 - T2) = (T2 - T0)(CP
- CV)
Here T2 is the temperature of the state with VB
and pA.
T2 = pBVAB/R = pAT0/PB.
Therefore ΔQβ
= T0(pA/pB - 1)(CP - CV).
Since Cp > Cv, heat is absorbed in each case.
(d) |Qβ| > |Qα|
The internal energy of the system is the same at points A and B since
the temperature is the same. The heat absorbed along a path is
therefore equal to the work done by the system along that path, Wβ
= pA(VB - VA), Wα = pb(VB
- VA),
|Qβ|/|Qα| = Wβ/Wα = pA/pB.
Problem:
A horizontal insulated cylinder is partitioned by a frictionless insulated
piston which prevents heat exchange between the two sides. On each side of the
piston we have 30 liters of an ideal monatomic gas at a pressure of 1 atmosphere
and a temperature of 300 K. Heat is very slowly injected into the gas on the
left, causing the piston to move until the gas on the right is compressed to a
pressure of 2 atmosphere.
(a) What are the final volume and temperature on the right side?
(b) What are the final values of P, V, and T on the left side?
(c) What is the change in the internal energy for the gas on the right side?
How much heat did it absorb and how much work was done on it?
(b) What is the change in the internal energy for the gas on the left side?
How much heat did it absorb and how much work was done on it?
Solution:
- Concepts:
Ideal gas law: PV = nRT, work done by the system: W = +∫PdV
Energy conservation: ΔU = ΔQ - ΔW, adiabatic processes: PVγ =
constant
- Reasoning:
The gas on the right side is compressed adiabatically. The pressure on both
sides is the same and the total volume of the left plus the right side is
constant.
- Details of the calculation:
(a) Since the compression is adiabatic, PVγ = constant.
For an ideal monatomic gas γ = 5/3.
Vf r= Vi(Pi/Pfr)1/γ = 30
liter * (0.5)3/5 = 19.8 liter ≈ 20 liters.
From the ideal gas law Tγ/Pγ-1 = constant, Tf r=
Ti(Pfr/Pi) γ-1/γ = 300 K * 22/5
≈ 396 K.
(b) The mechanical equilibrium implies Pf = 2 atm, the same as on
the right side.
Vfl = 2Vi - Vfr ≈ 40 liters. Then, from the
ideal gas law, Tfl = 300 K*(PV)fl/(PV)i ≈ 800
K.
(c) ΔU = (3/2)nRΔT. nR = PiVi/Ti. ΔU =
(3/2)PiVi(Tfr - Ti)/Ti ≈
14.4 atm-liter.
The gas on the right side did not absorb any heat, since it was compressed
adiabatically.
ΔQ = 0, ΔW = -14.4 atm-liter. Work is done on the gas.
(d) For the left side ΔU = (3/2)nRΔT = (3/2)PiVi(Tfl
- Ti)/Ti ≈ 75 atm-liter.
ΔW = 14.4 atm-liter, since the left and right side are always in mechanical
equilibrium.
The gas does work.
ΔQ = ΔU + ΔW ≈ 75 atm-liter is the amount of heat absorbed by the gas.
Let CP and CV denote the molar specific heat of an
ideal gas at constant pressure and at constant volume, respectively. Show that
CP - CV = R.
Let CP and CV denote the molar specific heat of an
ideal diatomic gas at room temperature at constant pressure and constant volume,
respectively. Find the ratio CP/CV.







