Thermal expansion and conductivity
Problem:
A mercury thermometer has a bulb of volume 0.100 cm3 at 10oC. The
capillary tube above the bulb has a cross-sectional area of 0.012 mm2.
The volume thermal expansion coefficient of mercury is 1.8*10-4 (oC)-1.
How much will the mercury rise when the temperature rises by 20oC?
Solution:
- Concepts:
The volume expansion coefficient
The average volume expansion coefficient b is defined through ΔV = βVΔT.
- Reasoning:
The volume of the mercury will increase as the temperature rises and the
mercury will rise in the capillary tube.
- Details of the calculation:
The increase in temperature is 20oC. The change in the volume of the
mercury is
ΔV = βVΔT = (1.8*10-4 (oC)-1)(0.100 cm3)(20
oC) = 3.6*10-4 cm3 = 0.36 mm3.
Δh = ΔV/A = (0.36 mm3)/(0.012 mm2) = 30 mm.
The mercury will rise 30 mm.
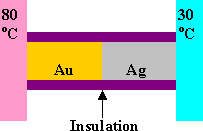
Problem:
A bar of gold is in thermal contact with a bar of silver of the same length
and area. One end of the compound bar is maintained at 80 oC and the
opposite end is at 30 oC. When the heat flow reaches steady state,
find the temperature at the junction.
The thermal conductivity of gold is 314 W/(moC), and the thermal
conductivity of silver is 427 W/(moC).
Solution:
- Concepts:
Thermal conductivity
The thermal conductivity k is defined through the equation
ΔQ/Δt = -kA
ΔT/Δx.
- Reasoning:
When a steady state is reached, then
the same amount of heat
crosses any cross sectional area perpendicular to the bar per second.
(Otherwise the energy would increase in certain regions, the temperature
would increase there, the temperature gradient would change, and we would
not have a steady state.) The amount of heat flowing is determined by the
temperature difference and the thermal conductivity.
- Details of the calculation:
If the temperature at the junction is T, then in the gold we have
ΔQ/Δt = (314 W/(moC))*A*(80 oC - T)/(L/2)
and in the silver we have
ΔQ/Δt = (427 W/(moC))*A*(T - 30 oC)/(L/2).
We can therefore write
(314 W/(moC))*A*(80 oC - T)/(L/2) = (427 W/(moC))*A*(T
- 30oC)/(L/2).
(314 W/(moC))*(80 oC - T) = (427W/(moC))*(T
- 30 oC).
(80 oC - T) = 1.36*(T - 30 oC).
120.8 oC = 2.36 T.
T = 51.2 oC.
Problem:
A Thermopane window of area 6 m2 is constructed of two layers of
glass, each 4 mm thick, separated by an air space of 5 mm. If the inside is at
20 oC and the outside is at -30 oC, what is the rate of
heat loss through the window?
Thermal conductivity of Thermopane: 0.8 W/(moC)
Thermal conductivity of air: 0.0234 W/(moC)
Solution:
- Concepts:
Thermal conductivity
The thermal conductivity k is defined through the equation.
- Reasoning:
When a steady state is reached, then the same amount of heat
crosses any cross sectional area perpendicular to the window per second.
(Otherwise the energy would increase in certain regions, the temperature
would increase there, the temperature gradient would change, and we would
not have a steady state.) The amount of heat flowing is determined by the
temperature difference and the thermal conductivity.
ΔQ/Δt = -kA
ΔT/Δx.
- Details of the calculations
When a steady state is reached, then the same amount of heat crosses any
cross sectional area per second. Let the temperature of the inner glass-air
boundary be T1 and the temperature of the outer glass-air
boundary be T2. Then for the inner piece of glass we have
ΔQ/Δt = (0.8 W/(moC))*6 m2*(20 oC - T1)/0.004
m.
For the air layer we have
ΔQ/Δt = (0.0234 W/(moC))*6 m2*(T1 - T2)/0.005
m.
For the outer piece of glass we have
ΔQ/Δt = (0.8 W/(moC))*6 m2*(T2 + 30 oC)/0.004
m.
We have 3 equations for three unknowns, ΔQ/Δt, T1, and T2.
The first and third equation yield (20 oC- T1) = (T2
+ 30 oC), T2 = -10oC - T1.
Inserting this expression for T2 into the second equation we have
ΔQ/Δt = (0.0234 W/(moC))*6 m2*(2 T1+10
oC)/0.005 m.
Combining this equation with the first equation then yields
(0.0234 W/(moC))*(2 T1+10 oC)/0.005 m =
(0.8 W/(moC))*(20o C - T1)/0.004 m.
0.0234*(2 T1+10 oC) = 20 oC - T1.
1.0468 T1 = 19.776 oC. T1 = 18.882 oC.
The first equation now yields
ΔQ/Δt = (0.8 W/(moC))*6 m2*(1.118 oC)/0.004
m = 1.34 kW.

