A tank having a volume of 0.1 m3 contains helium gas at 150 atm. How many balloons can the tank blow up, if each filled balloon is a sphere 0.3 m in diameter at an absolute pressure of 1.2 atm?
Solution:
A vertical open glass tube of length h is half-submerged in mercury. The top end of the tube is then closed and the tube is slowly pulled out until the bottom of the tube is barely submerged in the mercury. What is the length of the mercury column remaining in the tube? The atmospheric pressure corresponds to the pressure of a column of mercury of height H. Assume the temperature is constant.
Solution:
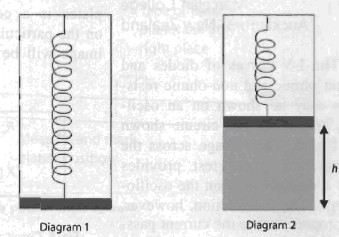
A movable heavy piston is supported by a spring inside a vertical cylindrical container as shown. When all air is pumped out of the container, the piston is in equilibrium, as shown in diagram 1, with only a tiny gap between the piston and the bottom of the container. When a portion of gas at temperature T is introduced under the piston, the piston rises to a height h as shown in diagram 2.

What would be the height of the piston above the bottom of the container if the gas was heated to temperature 2T? Assume the piston moves without friction and that the spring obeys Hooke's law.
Solution:
Near the surface of Earth where the gravitational acceleration has magnitude
g, derive an expression for the atmospheric pressure as a function of altitude y
assuming that
(a) the temperature T is constant.
(b) the temperature T is not constant, it decreases with altitude y. In
the standard atmosphere T = T0 - ay, with a = 0.0065 K/m.
Assume the atmosphere is an ideal gas of molecules with particle mass m.
Solution:
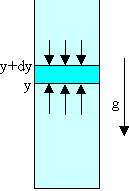
The pressure times the area (i.e. the force) at height y must exceed
that at height y + dy by the weight of the intervening gas.
We need Py+dyA - PyA = -mρparticleVg = -mρparticlegAdy,
or dP = -mρparticlegdy.
The ideal gas law lets us express ρparticle in terms of the
temperature and the pressure.
(a) By considering the forces acting on a thin concentric shell of atmosphere at a distance r from the center of the earth, show that the variation of pressure P with height in terms of the local density of the atmosphere ρ and the local gravitational acceleration g is given by
dP/dr = -ρg.
(b) If the temperature is 27o C at the earth's surface and the atmosphere is assumed to be an ideal gas composed approximately of 80% diatomic 14N and 20% diatomic 16O, estimate the vertical distance over which the pressure falls to 1/e of its value at the surface (this is called the scale height of the atmosphere). To simplify your estimate you may assume the temperature to remain constant with height above the surface (which typically introduces an error of ~20% compared to a more realistic variation of temperature).
Solution:
Consider a circular cylinder of radius R and length L, rotating about
its symmetry axis with angular velocity ω and containing an ideal gas with
particles of mass M. We assume that the system is in thermal equilibrium at
temperature T = 300 K, that the gas is at rest in a reference frame rotating
with the cylinder, and that the particle velocities are small enough that we can
ignore all the Coriolis forces.
(a) If P(0) is the pressure on the axis of
rotation, find the pressure P(r) between r = 0 and r = R.
(b) If R = 1 km, the gas consists of Nitrogen molecules, ω = 0.2/s, and P(R)
= 101 kPa, find the pressure on the axis.
(c) If R = 0.1 m, the gas consists of Nitrogen molecules, ω = 0.2/s, and P(R)
= 101 kPa, find the pressure on the axis.
Solution: