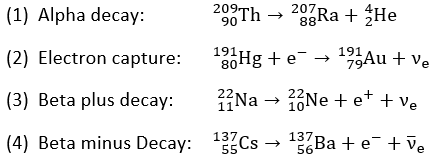The
Bohr atom, nuclear decay
Bohr atom
Problem:
Consider a Bohr-type, classical model of a hydrogenic atom, where a
structureless electron with charge -qe is orbiting a (stationary)
structureless nucleus with charge Zqe in a circular orbit in the xy-plane
without radiating. Let the angular momentum vector of the electron be
L
= Lk and have magnitude L = nħ.
(a) What is the orbital radius r0 of the electron?
(b) What is the kinetic energy of the electron and what is its total energy?
(c) What is the magnetic dipole moment μ of the atom?
Solution:
- Concepts:
The classical Bohr atom
- Reasoning:
The Bohr atom introduces angular momentum quantization, but otherwise is a
classical model.
- Details of the calculation:
(a) kZqe2/r02 = mv2/r0,
mvr0 = nħ, r0 = n2ħ2/(mkZqe2).
Let α = kZqe2. r0 = L2/(mα).
(b) ½mv2 = ½α/r0 = mα 2/(2n2ħ2).
Etotal = T + U = -½α/r0 = -m(kZqe2)2/(2n2ħ2).
(c) μ = IA
n = -(qev/(2πr))(πr2)
n =
= -½qevr = -(qe/(2m))L = -μBn
k.
Nuclei and particles
Problem:
Calculate the binding energy
of the deuteron, which consists of a proton and a neutron, given that the mass
of a deuteron is 2.013553 u.
Solution:
- Concepts:
Nuclear binding energy
- Reasoning:
The binding energy represents how much
energy we would have to supply to pull the nucleus apart into separate free
nucleons.
- Details of the calculation:
Binding energy: (mp + mn)c2 - mdc2
= 2.2 MeV
Problem:
Calculate the disintegration energy Q for the beta decay 32P --> 32S
+ e- + ν.
Atomic masses: m(32P) = 31.97391 u, m(32S) = 31.97207 u
Solution:
- Concepts:
Nuclear disintegration energy
- Reasoning:
Atomic masses include the masses of the atomic electrons. In β-
decay subtracting the atomic masses automatically takes into account the
mass of the emitted electron. This is not true for β+ decay.
The mass of the neutrino is negligibly small.
- Details of the calculation:
Q = mic2 - mfc2.
mi = mnuc(32P) = m(32P) - 15*m(e-),
mf = mnuc(32S) + m(e-) = m(32S)
- 16*m(e-) + m(e-) = m(32S) - 15*m(e-)
Q = mic2 - mfc2 = (m(32P)
- m(32S))c2 = 1.71 MeV.
Problem:
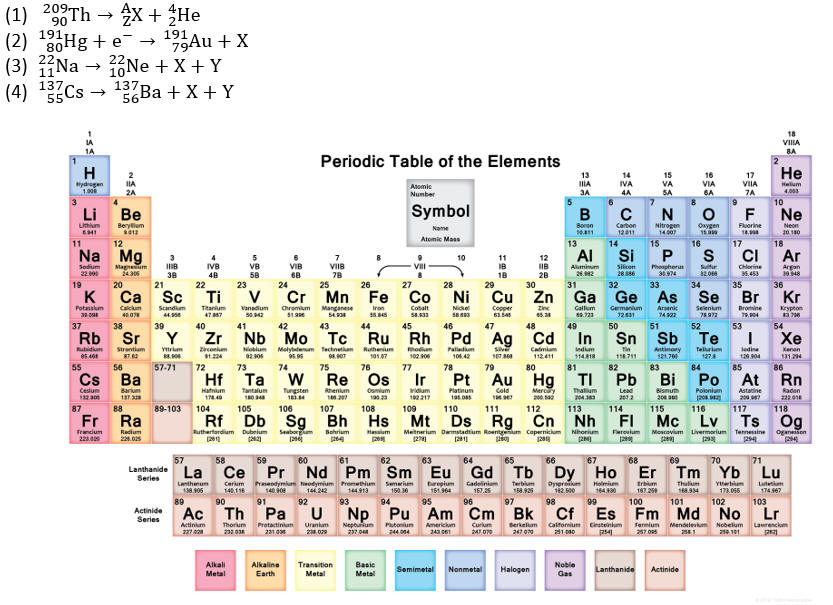
Complete the decays by replacing 'X' (and 'A', 'Z','Y', where relevant), and
state the name of the decay mode
Solution:
-
Concepts:
Nuclear decay modes
-
Reasoning:
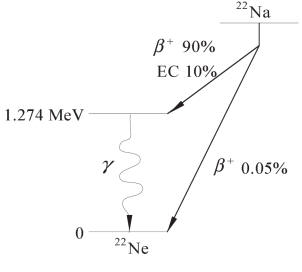
Aside:
10% of the time 22Na decays via electron capture, which
produce an excited state of 22Ne, which then decays by
emission of a 1.274 MeV gamma ray.
Problem:
Consider the case of a chain of two radioactive decays. Nucleus
A decays into another
B by one process, then
B decays into another
C by a second process, i.e.
A → B → C.
The mean life of A is τA = 1/λA, and the mean life of B is
τB = 1/λB,
Initially N0A nuclei of type A are present and N0B = 0.
(a) Find the decay rate (the number of decays per second) for B in terms of N0A,
λA, and λB,
(b) Find approximate expressions for the decay rate if
(i) λA >> λB, (ii) λA << λB,
(iii) λA ≈ λB,
Solution:
- Concepts:
Radioactive decay
- Reasoning:
The decay of an unstable nucleus is a random (probabilistic) process. The rate
at which nuclei decay is proportional to the number N of nuclei present.
- Details of the calculation:
(a) dNA/dt = - λANA, NA(t) = N0Aexp(-λAt).
dNB/dt = λANA - λBNB.
(feed rate plus decay rate)
dNB/dt = λAN0Aexp(-λAt) - λBNB.
Try a solution NB = C exp(-λAt) + D exp(-λBt).
D = - C since NB(0) = 0.
NB = C(exp(-λAt) - exp(-λBt)),
dNB/dt = -C(λAexp(-λAt) - λBexp(-λBt))
= λAN0Aexp(-λAt) - λBC(exp(-λAt)
- exp(-λBt)),
-CλA = λAN0A - λBC, C = λAN0A/(λB
- λA).
NB(t) = [λAN0A/(λB - λA)](exp(-λAt)
- exp(-λBt)).
Decay rate: RB = -λBNB = -[λBλAN0A/(λB
- λA)](exp(-λAt) - exp(-λBt)).
(b) (i) If λA >> λB, RB(t) ≈ -λBN0Aexp(-λBt).
The mean life of A is very short compared to the mean live of B, and nearly all
A nuclei have decayed into B nuclei in a time interval very much smaller than τB.
(ii) λA << λB, RB(t) ≈ -λAN0Aexp(-λAt).
The mean life of A is very long compared to the mean live of B. When a nucleus
A decays, the nucleus B that was formed is likely to decay in a time interval
very much smaller than τA.
(iii) λA ≈ λB, write λA = λ, λB
≈ λ + ε.
RB ≈ -[λ2N0A/ε](exp(-λt) (1 - 1 + εt) = -λ2N0At(exp(-λt).
As ε
--> 0 this becomes the exact solution for λA = λB = λ.
Problem:
A 1 MeV neutron beam is incident on an boron (20% 105B
+ 80% 115B) target of thickness 2 cm. The density of
the boron target is ρ = 2.3 g/cm3. The total cross section σtot
for interacting with the target (scattering plus attenuation) is 2.68 barns per atom
for 105B and 2.13 barns per atom for 115B.
Find the ratio of the transmitted flux to the incident flux, i.e. the
attenuation of the neutron beam.
Solution:
- Concepts:
Cross section and attenuation coefficient
- Reasoning:
F = F0exp(-μx), σ(cm2)*N(atoms/cm3)
= μ = attenuation coefficient.
(Assume the target is 20% 105B by weight.)
- Details of the calculation:
μ = 0.2*(2.68 barns/atom)*(10-24 cm2/barn)(6*1023 atoms/mole)*(1
mole/10 g)*(2.3 g/cm3)
+ 0.8*(2.13 barns/atom)*(10-24 cm2/barn)(6*1023 atoms/mole)*(1
mole/11 g)*(2.3 g/cm3)
= 0.288/cm.
F = F0exp(-0.288*2) = 0.56 F0.
Problem:
The dominant interactions between nucleons in a nucleus are the short range
nuclear force and the long-range electrostatic force. Nucleons are only
attracted by other nucleons in their neighborhood, but protons are repelled by
all other protons in the nucleus. When a large nucleus fissions, the net
attractions between nucleons stays roughly the same, while the net repulsion
decreases significantly.
To get a feeling for the amount of energy released in a fission reaction,
calculate the electrostatic potential energy of two fragments, say a Cesium and
a Zirconium nucleus when separated by a distance 2D0, twice the range
of the nuclear force.
(D0 is approximately 4 times the proton
diameter, D0 = 4*1.4*10-15 m.)
Solution:
- Concepts:
Electrostatic potential energy
- Reasoning:
The electrostatic potential energy of two charges Q1 and Q2 separated
by a distance r is U = kQ1Q2/r, where k = 9*109 Nm2/C2.
- Details of the calculation:
For Cesium Z = 55 and for Zirconium Z = 40.
U = (9*109 Nm2/C2)(55*40*(1.6*10-19)2 C2)/(2*4*1.4*10-15 m)
= 4.5*10-11J = 282 MeV.