Eigenvalues and Boltzmann statistics
Problem:
Due to the presence everywhere of the cosmic background radiation the minimum
possible temperature of a gas in interstellar space is 2.7 K. This implies that
a significant fraction of molecules in space may be in low-level excited
states. Consider a hypothetical molecule with one possible excited state. What
would the excitation energy have to be for 20% of the molecules to be in the
excited state?
Solution:
- Concepts:
Boltzmann statistics
- Reasoning:
Boltzmann statistics tells us that Nexcited/Nground =
e-ΔE/kT, if the degeneracy of both states is 1.
- Details of the calculation:
0.2/0.8 = exp(-ΔE/(kT))
ln(0.25) = -ΔE/(kT)
ΔE = -ln(0.25)*(kT) = -ln(0.25)*8.617*10-5*2.7 eV = 3.2*10-4
eV = 0.32 meV
Problem:
Consider a particle in a simple two-level system with eigenstates ψ1
and ψ2 such that
Hψ1 = E1ψ1 and Hψ2 = E2ψ2.
Derive an expression for the specific heat per particle cV = ∂<E>/∂T of the
system as a function of temperature.
Solution:
- Concepts:
Boltzmann statistics
- Reasoning:
The system consisting of many particles is in a statistical mixture of
states.
In thermodynamic equilibrium P(Ei) ∝ giexp(-Ei/(kT)
Here the degeneracy gi is 1 for all i.
- Details of the calculation:
Average energy: <E> = (E1 exp(-E1/(kT)) + E2
exp(-E2/(kT)))/(exp(-E1/(kT)) + exp(-E2/(kT)))
<E> = (E1 exp(-βE1) + E2 exp(-βE2))/(exp(-βE1)
+ exp(-βE2))
= (E1 + E2 exp(-β∆))/(1 + exp(-β∆)),
with β = 1/(kT) and ∆ = E2 - E1.
cV = ∂<E>/∂T. cV = (∂<E>/∂β)(∂β/∂T) = -(1/(kT2))∂<E>/∂β.
cV = [1/(kT2)][(E2∆exp(-β∆))/(1 + exp(-β∆)) -
(E1 + E2exp(-β∆))(∆exp(-β∆)))/(1 + exp(-β∆))2].
Problem:
Consider a system of N particles with only 3 possible energy levels separated
by ε. Let the ground state energy be zero. The system occupies a
fixed volume V and is in thermal equilibrium with a reservoir at temperature T.
Ignore interactions between particles and assume Boltzmann statistics apply.
(a) What is the partition function for a single particle in the system?
(b) What is the average energy per particle?
(c) What is the probability that the 2ε level is occupied in the
high-temperature limit kBT >> ε?
Explain your answer on physical grounds.
(d) What is the average energy per particle in the high-temperature limit kBT
>> ε?
(e) At what energy is the ground state 1.1 times as likely to be occupied
as the 2ε level?
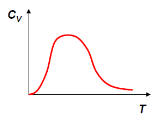
(f) Find the heat capacity CV of the system, analyze the low-T
(kBT >> ε) and high-T (kBT >> ε) limit, and sketch CV as a
function of T.
Solution:
- Concepts:
Boltzmann statistics
- Reasoning:
In thermodynamic equilibrium P(Ei) ∝ giexp(-Ei/(kT).
Here the degeneracy gi is 1 for all i.
- Details of the calculation:
(a) P(E) = Cexp(-E/(kBT)).
ΣjP(Ej) = CΣjexp(-Ej/(kBT))
= 1, C = 1/Σjexp(-Ej/(kBT)) = 1/Z.
Let β = 1/(kBT)).
Z = 1 + exp(-βε) + exp(-2βε).
(b) <ε> = (ε exp(-βε) + 2ε exp(-2βε))/Z
= ε(exp(-βε) + 2exp(-2βε))/(1 + exp(-βε) + exp(-2βε)).
(c) P = exp(-2βε)/(1 + exp(-βε) + exp(-2βε)) ~ (1 - 2βε)/( 1 + 1 - βε + 1 - 2βε)
= 1/3.
In the high energy limit the probability is the same for all three levels.
(d) <ε> = 0/3 + ε/3 + 2ε/3 = ε.
(e) exp(-2βε) = 1/1.1, 2βε = ln(1.1), T = 2ε/(kB*ln(1.1)).
(f) CV = dU/dT = Nd<ε>/dT = Nd<ε>/dβ)(dβ/dT).
CV = (Nε2/(kBT2))[ exp(-βε) + 4
exp(-2βε) + exp(-3βε)]/[1 + exp(-βε) + exp(-2βε)]2.
Low temperature limit: CV = (Nε2/(kBT2))
exp(-ε/(kBT)). (CV --> 0 as T --> 0)
High temperature limit: CV = (2/3)(Nε2/(kBT2)).
( CV --> 0 as T --> ∞)