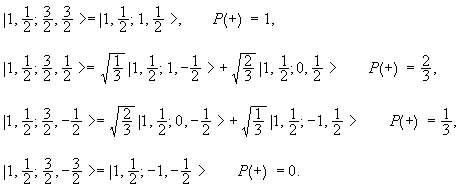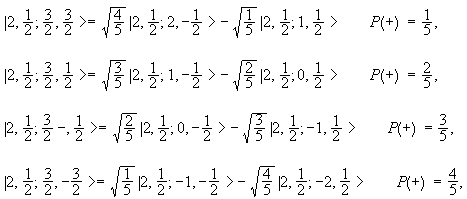Addition of angular momentum
Problem:
You have a system of two electrons whose orbital quantum
numbers are l1 = 2 and l2 = 4
respectively.
(a) Find the possible values of l (total orbital angular momentum
quantum number) for the system.
(b) Find the possible values of s (total spin angular momentum quantum number)
for the system.
(c) Find the possible values of j (total angular momentum quantum number)
for the system
Solution:
- Concepts:
Addition of angular momentum
- Reasoning:
We are supposed to add the orbital and spin angular momentum.
- Details of the
calculation:
(a) The quantum numbers associated with the total orbital angular
momentum will range from a maximum value found by adding the individual
quantum numbers together to a minimum value found by taking the absolute
value of the difference of the two numbers in integer steps.
lmax = l1 + l2 = 2
+ 4 = 6
lmin = |l1 - l2|
= |2 - 4| = 2
The possible values for L are 2, 3, 4, 5, and 6 .
(b) The quantum numbers associated with the total spin angular momentum
will range from a maximum value found by adding the individual quantum
numbers together to a minimum value found by taking the absolute value of
the difference of the two numbers in integer steps.
smax = s1 + s2 = ½ +
½ = 1
smin = |s1 - s2| = |½ -
½| = 0
The possible values for s are 0 and 1.
(c) The largest possible value for j will be found by adding together the
maximum values for both l and s. The minimum value for j will be
found by subtracting the largest possible value of s from the smallest
possible value for l.
jsmax = lmax + smax = 6 + 1 = 7
jmin = |lmin - smax| = |2 - 1| = 1
j = 1, 2, 3, 4, 5, 6, 7
Problem:
A hydrogen atom is known to be in a state characterized the
quantum numbers n = 3, l
= 2.
(a)
Give the allowed values of j.
(b)
For each of the allowed values of j, calculate the square of the
magnitude of the total angular momentum.
Solution:
- Concepts:
Addition of angular momentum
- Reasoning:
We are supposed to add the
orbital and spin angular momentum of the electron in the hydrogen atom.
- Details of the calculation:
(a)
l = 2, s = ½, j = 5/2, 3/2.
(b)
J2|jm> = j(j + 1)ħ2|jm>.
For j = 5/2, j(j + 1)ħ2
= (35/4)ħ2.
For j = 3/2, j(j + 1)ħ2
= (15/4)ħ2.
Problem:
Consider a deuterium atom (composed of a nucleus of spin 1 and an electron).
The electronic angular momentum is J =
L +
S, where L
is the orbital angular momentum of the electron and S is its spin. The
total angular momentum of the atom is F =
J +
I, where I
is the nuclear spin. The eigenvalues of J2 and F2 are
j(j + 1)ħ2 and f(f + 1)ħ2
respectively.
(a) What are the possible values of the quantum numbers j and f for a deuterium
atom in the 1s ground state?
(b) What are the possible values of the quantum numbers j and f for a deuterium
atom in the 2p excited state?
Solution:
- Concepts:
Addition of angular momenta
- Reasoning:
We are asked to add three
angular momenta
- Details of the calculation:
(a) For the 1s ground state we have l = 0, J =
L
+ S = S, j = ½.
F = J +
I, i = 1.
The possible values for f are f = i + j, i - j; f = 3/2, ½.
(b) For the 2p state we have l = 1.
The possible values for j are j = l + s, l - s; j = 3/2, ½.
If j = 3/2 then the possible values for f are
f = j + i, j + i - 1, j - i; f = 5/2, 3/2, ½.
If j = ½ then the possible values for f are
f = i + j, i - j; f = 3/2, ½.
So the possible values for f are f = 5/2, 3/2, ½.
Problem:
We are to add two angular momenta
characterized by the quantum numbers
j1 = 2 and j2 = 1.
(a) What are the possible values for j?
(b) Express all eigenkets |j1, j2;
j, m> = |2, 1; 1, m> in terms of |j1, j2; m1, m2>.
(c) Express the ket |j1, j2; m1,
m2> =|2, 1; 0, 0> in terms of |j1, j2; j, m>.
(d) What are the expectation values of J1z
and J2z in the state |j1, j2; j, m> = |2, 1; 1,
1>?
Solution:
- Concepts:
Addition of angular momentum, the Clebsch-Gordan coefficients
- Reasoning:
Consider two angular momentum
operators J1 and
J2.
J1
operates in E1 and J2
operates in E2.
Let J = J1
+J2. J operates in E =
E1⊗E2.
Since the operators J12, J1z , J22,
and J2z all commute, a basis of common eigenvectors for
E exists.
We denote this basis by {|j1, j2; m1, m2>}.
Since the operators J12, J22, J2,
and Jz all commute, a basis of common eigenvectors for
E exists.
We denote this basis by {|j1, j2; j, m>}.
We can write the vectors of one basis as linear combinations of the vectors of
the other basis.
|j1,j2;j,m> = ∑m1∑m2 Cjm1m2|j1,j2;m1,m2>,
|j1,j2;m1,m2> = ∑j∑m
Cjm1m2|j1,j2;j,m>.
The Cjm1m2 = <j1,j2;m1,m2|j1,j2;j,m> are called the
Clebsch-Gordon
coefficients.
- Details of the calculation:
(a) |j1 - j2| ≤ j ≤
j1 + j2. j = 3, 2, 1.
(b) |j1, j2; j, m>
Using a table of Clebsch-Gordan coefficients we find:
|2, 1; 1, 1> = (3/5)½|2, 1; 2, -1> - (3/10)½|2, 1; 1, 0>
+ (1/10)½|2, 1; 0, 1>
|2, 1; 1, 0> = (3/10)½|2,1;1 ,-1> - (2/5)½|2, 1; 0, 0>
+ (3/10)½|2, 1; -1, 1>
|2, 1; 1, -1> = (1/10)½|2,1;0,-1> - (3/10)½|2, 1; -1, 0>
+ (3/5)½|2, 1; -2, 1>
(c)
|j1, j2; m1, m2>
Using a table of Clebsch-Gordan coefficients we find:
|2, 1; 0, 0> = (3/5)½|2, 1; 3, 0> - (2/5)½|2, 1; 1, 0>
(d) Using the result from part (b) we have
<J1z> = 2ħ(3/5)
+ ħ(3/10) = 3ħ/2,
<J2z> = -ħ(3/5)
+ ħ(1/10) = -ħ/2.
Problem:
A particle of spin 3/2, at rest in the laboratory, disintegrates into two
particles, one of spin ½ and one of spin 0.
(a) What values are possible for the relative orbital angular momentum of
the two particles? Show that there is only one possible value if the
parity of the relative orbital state is fixed.
(b) Assume the decaying particle is initially in the eigenstate of Sz
with eigenvalue mħ.
Is it possible to determine the parity of the final state by measuring the
probabilities of finding the spin ½ particle either in the |+> or |-> state?
Solution:
- Concepts:
Conservation of angular momentum, addition of angular momentum, the
Clebsch-Gordan coefficients,
orbital angular momentum and parity
- Reasoning:
The total angular momentum of an isolated system is conserved. The
initial state has only spin angular momentum. The spin angular momenta and the
orbital angular momentum of the particles in the final state must add to give
the angular momentum of the initial state.
-
Details of the calculation:
(a) The initial value of j is j = 3/2. We need the final value to
be j = 3/2, since angular momentum of an isolated system is conserved.
Let us call the initial particle particle a, the spin ½ particle particle b,
and the spin 0 particle particle c. Then the total spin of the two
particles in the final state is S = Sb + Sc
= Sb. Therefore the spin quantum number is s = ½. The
possible values for the orbital angular momentum quantum number are l = 1 and
l = 2.
(j = l + s, ..., l - s; j = l + ½, l - ½, implies l = 1 or l = 2.) The
parity of the orbital state is (-1)l. If the parity is odd,
we have l = 1, if the parity is even, we have l = 2.
(b) Assume that the final state has l = 1.
It may be written as |j1, j2; j, m> = |1, ½; 3/2, m>.
We may write it as a linear combination of |j1, j2; m1,
m2> = |1, ½; ml, ms >.
m can take on the values 3/2, ½, -½, -3/2. Using a table of
Clebsch-Gordan coefficients we find
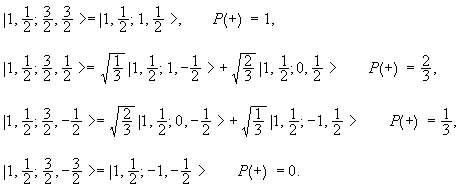
Here P(+) is the probabilities of finding the spin ½ particle in the |+>
state.
Now assume that the final state has l = 2.
It may be written as |j1, j2; j, m> = |2, ½; 3/2, m>.
We may write it as a linear combination of |j1, j2; m1,
m2> = |2, ½; ml, ms >.
m can take on the values 3/2, ½, -½, -3/2. We find
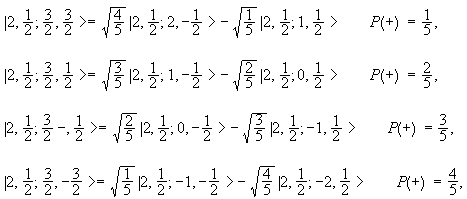
Yes, it is possible to determine the parity of the final state by measuring
the probabilities of finding the spin ½ particle either in the |+> or |->
state.