The hydrogen atom
Energy levels
Problem:
What is the ratio of the longest wavelength of the Balmer series to the
longest wavelength of the Lyman series?
Solution:
- Concepts:
The hydrogen atom
- Reasoning:
hc/λ = 13.6 eV(1/nf2 - 1/ni2)
Longest wavelength <--> smallest energy difference
- Details of the calculation:
For the Balmer series hc/λmax = 13.6 eV(¼ - 1/9) (n = 3
--> n = 2).
For the Lyman series: hc/λmax = 13.6 eV(1/1 - ¼) (n = 2 --> n
= 1).
λmax(Balmer)/λmax(Lyman) = (1/1 - ¼) /(¼ - 1/9) =
27/5.
Problem:
A hydrogen atom at rest in the laboratory emits the Lyman α radiation.
(a) Express the measured wavelength in terms of the Rydberg constant RH
= 1.097*107/m, which was determined from measurements.
(b) Compute the recoil kinetic energy of the atom.
(c) What fraction of the excitation energy of the n = 2 state is carried
by recoiling atom.
Solution:
- Concepts:
The hydrogen atom, momentum conservation
- Reasoning:
The Rydberg constant was determined from measurements. The measured
wavelength of the photon is NOT the wavelength we calculate assuming a fixed
atom.
- Details of the calculation:
(a) Lyman α radiation is produced in a n = 2 to n = 1
transition.
1/λ = RH(1 - ¼) = 3RH/4, λ = 1.22*10-7
m.
The energy released in the transition is E = 13.6 eV(1 - ¼) = 10.2 eV.
(b) Momentum conservation requires that |pphoton| = |patom|
= p, h/λ = p.
The recoil energy of the atom is p2/(2m) = (0.75 h RH)2/(2m)
= 8.9*10-27 J = 5.58*10-8 eV.
(c) The fraction of the excitation energy carried by the atom is
5.58*10-8/10.2 = 5.5*10-9.
Problem:
In the interstellar medium electrons may recombine with protons to form
hydrogen atoms with high principal quantum numbers. A transition between
successive values of n gives rise to a recombination line.
(a) A radio recombination line occurs at 5.425978 *
1010 Hz for a n = 50 to n = 49 transition. Calculate the Rydberg constant for H.
(b) Compute the frequency of the H recombination line corresponding to
the transition n = 100 to n = 99.
(c) Assume the mean speed in part (b) is 106 m/s. At what
frequency or frequencies would the recombination line be observable?
(d) Consider that radio recombination lines may be observed at either of two
facilities, the 11 meter telescope at Kitt Peak near Tuscon, Arizona, and the
1.2 meter radio telescope at Columbia University in New York. Relative larger
blocks of time are available on the smaller telescope, but its intrinsic noise
is moderately high. Where would you choose to map recombination radiation
emanating from an external galaxy. Discuss both technical and non technical
aspects of your choice.
Solution:
- Concepts:
The hydrogen atom, transitions between energy levels, the Doppler shift
- Reasoning:
The wavelengths of the light emitted by excited hydrogen atoms can be found
using the formula
1/λ = RH(1/n2 - 1/n'2). The observed
wavelengths depend on the relative speed of the source and the observer.
-
Details of the calculation:
(a) En' - En
= hν = hcRH(1/n2 -
1/n'2).
5.425978 *
1010 = 2.99792458 *
108RH(¼92 - 1/502), RH
= 1.097373 *
107 m-1.
(b) ν = cRH(1/992
- 1/1002) = 6.679710 *
109 s-1.
(c) The line is Doppler shifted.
ν'
= ν[(1
+ v/c)/(1 - v/c)]½ ~ ν(1
+ v/c) if v/c << 1.
Here v is the relative velocity of source and observer; v is positive
if the source and the observer approach each other, and v is negative if the
source and the receiver recede from each other.
-106/(3*108) < v/c < 106/(3*108),
6.657 *
109 s-1 < ν' < 6.702 *
109 s-1.
(d) Here are some factors worth considering:
The amount of radiation gathered by a telescope is proportional to the
square of its diameter D.
The smallest angle that can be resolved is approximately θ = λ/D.
(For each photon we have ΔxΔpx
~ h, Δx ~ D, ΔPx
~ h/D, θ = Δpx/p
= hc/(Dhν) = λ/D.)
If you assume that the radiation has a frequency of ~ 10-10 s-1, then
λ = c/ν = 3 cm. For the small telescope we therefore
have θ = 0.03/1.2 while for
the large telescope we have θ = 0.03/11. It takes
(11/1.2)2 = 84 times as long to gather
the same amount of radiation with the small telescope as with the large
telescope.
Problem:
Determine the wavelengths of
the first three transitions in the Balmer series for hydrogen. How would the
wavelengths change for an ion with Z = 2 (He+)?
Solution:
- Concepts:
Hydrogenic atoms, The Balmer series
- Reasoning:
Each time the energy level of the electron
changes, a photon will be emitted and the energy (wavelength) of the photon
will be characteristic of the energy difference between the initial and
final energy levels of the atom in the transition. The lines for which nf
= 2 are called the Balmer series.
- Details of the calculation:
The final state for a Balmer-series
transition is n = 2.
hf = -13.6 eV(1/ni2 - ½2) = 13.6 eV(¼ -
1/ni2).
We may write hc/λ
= 13.6 eV(¼ - 1/ni2) or
1/λ
= (13.6 eV(/hc))(¼ - 1/ni2) = R(¼ - 1/ni2).
R = 1.1*10-2
nm-1.
n = 3 --> n = 2: 1/λ
= (1.1*10-2 nm-1)(¼ - 1/9),
λ = 655 nm
n = 4 -->
n = 2: 1/λ
= (1.1*10-2 nm-1)(¼ - 1/16),
λ = 485 nm
n = 5 -->
n = 2: 1/λ
= (1.1*10-2 nm-1)(¼ - ½5),
λ = 433 nm
Energy level and transition frequencies in hydrogenic atoms scale as
Z2, so for a Z = 2 ion the transition
wavelengths a factor of 4 smaller than those for hydrogen.
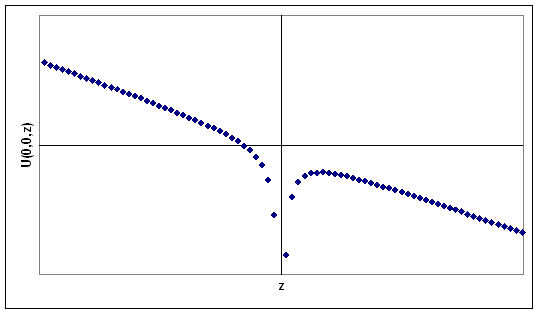
Problem:
A hydrogen atom is placed in a uniform electric field, E = -Ek.
Place the proton at the origin of your coordinate system. An electron in the
hydrogen atom then has potential energy U(r) = -kqe2/r
- qeEz. U(0,0,z) becomes increasingly positive for negative z . For
positive z the potential energy U(0,0,z) contains a "hill" and then decreases
with increasing z.
Sketch the potential energy of the electron, U(0,0,z), as function of z and
calculate the energy at the maximum at positive z. Equate this energy to the
energy of the unperturbed (zero field) hydrogen energy level and thereby
determine the value of the field required to field-ionize a hydrogen atom with
principle quantum number n (neglect tunneling).
Solution:
- Concepts:
The energy levels of the hydrogen atom
- Reasoning:
The perturbed potential has a local maximum. If an energy level of the
unperturbed hydrogen atom lies above this local maximum, than field
ionization is likely.
- Details of the calculation:
U(0,0,z) = - kqe2/|z| - qeEz.
For positive z:
dU(0,0,z)/dz = kqe2/z2
- qeE = 0. z2 = kqe/E, z = (kqe/E)½.
Let - kqe2/(kqe/E)½ - qeE(kqe/E)½
= -(EI)/n2, with EI = 13.6 eV.
Then -2qe(kqeE)½ = -(13.6 eV)/n2,
4kqe3E = EI2/n4,
E = EI2/(4kqe3n4) =
(3.21*1010 V/m)/n4.
A reasonable field (kV/cm) can ionize levels with n > 24.
Wave functions
Problem:
An electron in the n = 2, l = 1, m = 0 state of
a hydrogen atom has a wave function ψ(r,θ,φ)
proportional to r exp(-r/(2a0)) cosθ, where a0
is the Bohr radius.
(a) Find the normalized wave function
ψ(r,θ,φ).
(b) Find the probability per unit length of finding the electron a distance
r from the nucleus.
(c) Find the most probable distance RMP of the electron from
the nucleus.
(d) Find the average distance <r> from the nucleus.
Solution:
- Concepts:
The fundamental assumptions of
Quantum Mechanics
- Reasoning:
If the wave function is normalized, then |ψ(r,t)|2
is the probability density.
The expression for the mean value of any observable A in the normalized state |ψ> is
<A> = <ψ|A|ψ>.
-
Details of the calculation:
(a) Normalization:
∫|ψ(r,θ,φ)|2d3r = 1, ψ(r,θ,φ)
= A r exp(-r/(2a0)) cosθ.
A2∫02πdφ∫0πsinθ
dθ∫0∞r2dr r2exp(-r/a0) cos2θ
= A22π∫0πsinθ
cos2θ dθ∫0∞r4dr
exp(-r/a0) = 1.
A22πa05∫-11x2dx∫0∞r'4dr'
exp(-r') = A22πa05(2/3)4!' = 1.
A2 = 1/(32πa05).
ψ(r,θ,φ) = (32πa03)-½(r/a0) exp(-r/(2a0)) cosθ.
(b) P(r,θ,φ)d3r
= |ψ(r,θ,φ)|2d3r
is the probability of finding the electron in an infinitesimal volume d3r
about r =
(r,θ,φ).
P(r,θ,φ)
is the probability per unit volume.
∫02πdφ∫0πsinθ
dθ |ψ(r,θ,φ)|2r2 = P(r).
P(r) is the probability per unit length.
P(r) = [2π/(32πa05)] r4exp(-r/a0)∫0πsinθ
cos2θ dθ
= [r4/(16a05)] exp(-r/a0)(2/3) =
[r4/(24a05)] exp(-r/a0).
(c) We are looking for the maximum in P(r).
dP(r)/dr|(r = RMP) = [1/(24a05)] [4r3
exp(-r/a0) - (r4/a0)exp(-r/a0)]|(r
= RMP) = 0.
RMP = 4a0.
(d)
<r> =
∫0∞r P(r) dr = [1/(24a05)]
∫0∞r5 exp(-r/a0) dr
= [a0/24] 5! = 5a0.
<r> > RMP.
Problem:
Assume that the nucleus of a hydrogen atom is a sphere of
radius b. In the limit where
b << a0 (where a0 is the Bohr radius), what is the
probability that an electron in the ground state of hydrogen will be found
inside the nucleus?
Solution:
- Concepts:
Ground-state wave function of the hydrogen atom
- Reasoning:
The ground state wave function does not vary much over the volume of the
nucleus, since b << a.
- Details of the calculation:
The ground state wave function of the hydrogen atom is Φ100(r)
= (πa03)-½ exp(-r/a0).
The probability of finding the electron inside the nucleus is
P = Vn
|Φ100(0)|2 =
(4πb3/3) (πa03)-1 = (4/3)(b/a0)3.
Problem:
Find the momentum space wave function Φ(p) for the electron in the 1s
state of the hydrogen atom.
Solution:
- Concepts:
Change of representation, the Fourier transform
- Reasoning:
We are asked to change from the
r to the p representation.
Φ(r)
= (2πħ)-3/2∫
d3p Φ(p)exp(ip∙r/ħ),
Φ(p)
= (2πħ)-3/2∫d3r Φ(r)exp(-ip∙r/ħ).
Φ(r)
and Φ(p)
are Fourier transforms of each other.
- Details of the calculation:
The 1s wave function is
Φ(r) = (πa03)-½
exp(-r/a0) =
Φ(r).
Symmetry demands that Φ(p)
= Φ(p).
Let us choose a convenient direction for p. Let
p = pk.
Then
Φ(p)
= (2πħ)-3/2∫02πdφ∫0πsinθ
dθ∫0∞r2dr exp(-iprcosθ/ħ)
(πa03)-½exp(-r/a0)
= (2πħ)-3/2(πa03)-½2π∫-11dcosθ∫0∞r2dr
exp(-iprcosθ/ħ) exp(-r/a0).
∫0∞r2dr exp(-iprcosθ/ħ)
exp(-r/a0) = 2/(iprcosθ/ħ + 1/a0)3
using
∫0∞xn exp(-x) dx = n!.
Φ(p) = a-3/2ħ3/2(√2)(-ip3π)-1∫-11dx
(x + ħ/(ia0p))-3
= (a0ħ)-3/2ħ3(√2)(-ip3π)-1(2ħ/(ia0p))(1
+ ħ2/(a0p)2)-2.
Φ(p) = (1/π) (2a0/ħ)3/2 [1/(1 + a02p2/ħ2)2]
=
Φ(p).
Problem:
The normalized wave function of an electron in a hydrogen atom, neglecting
spin, is
ψ(r,t) = (1/3)½Φ100(r,θ,φ)
+ (2/3)½Φ210(r,θ,φ),
where the Φnlm(r,θ,φ)
are the usual normalized hydrogenic eigenfunctions.
(a) If a single measurement is made of the energy, what results are possible?
(b) What are the probabilities of obtaining each of the particular possible
results?
(c) What is the expectation value of the energy?
(d) If a single measurement is made of the total angular momentum, what results
are possible?
(e) What are the probabilities of obtaining each of the particular possible
results?
(f) If the observable O is a constant of the motion, then it satisfies the
equation
d/dt<O> = <∂O/∂t>
+ (i/ħ)<[H,O]> = 0
where H is the
Hamiltonian.
For the hydrogen atom described above, is the z-component of angular momentum, Lz,
a constant of the motion?
(g) Is the z-component of linear momentum, pz, a constant of the
motion?
(h) How do the expectation values of Lz and pz depend on
time?
Given:
Φ100(r,θ,φ)
∝ exp(-r/a0),
Φ210(r,θ,φ)
∝ (r/a0) exp(-r/(2a0)) cos(θ).
∫Φ*100
(∂/∂z)
Φ210 d3r = (√2/a0)(2/3)4
exp(-i(E2 - E1)t/ħ)
= A exp(-i(E2 - E1)t/ħ)
∫Φ*210
(∂/∂z)
Φ100 d3r = -(√2/a0)(2/3)4
exp(i(E2 - E1)t/ħ)
= -A exp(i(E2 - E1)t/ħ)
Solution:
- Concepts:
Postulates of Quantum Mechanics, the hydrogen atom
- Reasoning:
The postulates of quantum mechanic contain the rules for making predictions
about the outcome of measurements.
- Details of the calculation:
(a) The possible results are E1 = -13.6
eV or E2 = -13.6 eV/4 = -3.4 eV.
(b) P(E1) = (1/3), P(E2)
= (2/3).
(c) <E> = -13.6 eV/3 - 2*3.4eV/2 = 6.8.
(d) The possible results are L = 0 or L = (2ħ2)½.
(quantum number l = 0 or l = 1)
(e) P(L = 0) = (1/3), P(L = 1.41ħ)
= (2/3).
(f) H commutes with Lz. ψ(r,t)
is an eigenfunction of Lz with eigenvalue 0. Lz is a constant of motion.
(g) H does not commute with pz, pz
is not a constant of the motion.
(h) d<Lz>/dt = 0, since
Lz is a constant of motion.
<pz> = (ħ/i)
∫ψ*(r,t)(∂/∂z)ψ(r,t)d3r
= (ħ/i)[(1/3)∫Φ*100(∂/∂z)Φ100d3r
+ (2/3) ∫Φ*210(∂/∂z)Φ210d3r
+ (√2/3) ∫Φ*100(∂/∂z)Φ210d3r
+ (√2/3) ∫Φ*210(∂/∂z)Φ100d3r
]
= (√2/3)(ħ/i)[A(exp(-i(E2
- E1)t/ħ) -
A(exp(i(E2 - E1)t/ħ)]
= (-2Aħ)(√2/3)sin((E2
- E1)t/ħ)
[For any eigenstate of the hydrogen
atom <pz> = 0, therefore
∫Φ*100(∂/∂z)Φ100d3r
= ∫Φ*210(∂/∂z)Φ210d3r
= 0.]
Problem:
For l = 2 in the hydrogen atom:
(a) What is the minimum value of Lx2 + Ly2?
(b) What is the maximum value of Lx2 + Ly2?
(c) What is Lx2 + Ly2 for l = 2 and
ml = 1? Can either Lx or Ly be
determined from this?
(d) What is the minimum value of n this state can have?
Solution:
- Concepts:
Angular momentum, the hydrogen atom
- Reasoning:
Φnlm(r) = Rnl(r)Ylm(θ,φ)
for hydrogen.
L2Ylm(θ,φ) = l(l + 1)ħ2Ylm(θ,φ), LzYlm(θ,φ)
= mħYlm(θ,φ).
The quantum number m can take up values from -l to l in integer steps.
-
Details of the calculation:
(a) L2 = Lx2 + Ly2 + Lz2
= l(l + 1)ħ2 = 6ħ2. The maximum value of Lz is
2ħ, so the minimum value of Lx2 + Ly2
= L2 - Lz2 = 2ħ2.
(b) The minimum value of Lz is 0, so the maximum value of Lx2
+ Ly2 = 6ħ2.
(c) For l = 2 and ml = 1 Lz2 = ħ2,
so Lx2 + Ly2 = 5ħ2.
Neither Lx or Ly can be
determined from this?
(d) For the hydrogen atom the allowed value for l are all non-negative integers
equal to or less than n - 1. So the minimum value of n this state can have is 3.
