

In this laboratory you will study the absorption of the decay products of Cesium 137 and Strontium 90 in different materials.
Background information:
Radioactive decay is a random process. We cannot predict exactly when a certain unstable nucleus will decay, we can only predict the probability that the nucleus will decay in a certain time interval. For a large sample, the decay rate is approximately R = R0exp(-t/τ). If the mean life τ is very large and we make measurements for a time interval t << τ, then during that time interval the decay rate R is approximately constant. R ~ R0 = N0/τ.
In this experiment radiation is produced at an approximately constant rate. We let that radiation travel through materials of different mass thickness xm. The mass thickness of a material is measured in mass per unit area, for example g/cm2. It is just the thickness of the material (cm) times the density of the material (g/cm3).
Absorption of radiation is also a random process. When a particle travels through a material, we cannot predict exactly how far it will penetrate and at which depth it will be absorbed, we can only predict the probability that the particle will travel through a certain distance ∆x of the material.
When a beam of N0 particles crosses a layer of absorber of mass thickness xm, the number of particles that emerge is given by
N = N0 exp(-μmxm).
Here μm is called the mass attenuation coefficient of the material. The rate at which particles are removed from the beam is
dN/dxm = -μmN.
xm1/2 is the half-value for the mass thickness, and it is given by xm1/2 = ln(2)/μm.
The two most dangerous fission products in nuclear wastes are Strontium-90 (Sr-90) and Cesium-137 (Cs-137). Their half-life is long enough so one has to worry about storage of the materials but short enough so one has to worry about high radiation release. The two types of radioactive nuclei account for about 98% of the radioactive hazard after 10 years of storage. Both nuclei have similar half-lives of about a human generation. Sr-90's half-life is 28 years and Cs-137's is 30 years. This means that after storing these materials for 30 years, 50% of the nuclei will have transmuted into other elements.
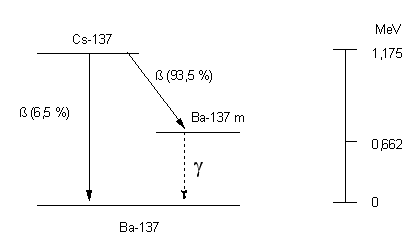
Cs-137 disintegrates with a probability of 6.5% directly and with a probability of 93.5% indirectly over the meta-stable barium-137m into stable barium-137. During the indirect decay, beta rays having a maximum energy of 0.513 MeV are released. The meta-stable Ba-137 changes into stable Ba-137 with a half-life of 2.55 minutes releasing a 0.662 MeV gamma ray. The activity the Cs-137 is is deduced from the gamma rays.
Sr-90 beta-decays (0.546 MeV) into yttrium-90, which has a half-life of 64 hours. Yttrium-90 beta-decays with a a probability of +99% directly into stable Zirconium-90 while emitting electrons with energies up to Emax = 2.27 MeV. The activity of the Sr-90 is is deduced from the beta rays.
Equipment needed:

Procedure:
Part 1:
You will measure xm1/2, the half-value of the mass thickness, for 0.662 MeV gamma rays in lead.
Part 2:
You will measure xm1/2, the half-value of the mass thickness, for Emax = 2.27 MeV beta rays from the decay of Sr-90 in polyethylene.
Data Analysis:
Part 1:
Part 2:
Open Microsoft Word and prepare a report.
Summarize the experiment.
Insert your graphs.
Discuss your results. In the discussion you should answer the following questions:
Which value did you obtain for xm1/2, the half-value of the mass thickness, for 0.662 MeV gamma rays in lead?
Which value did you obtain for xm1/2, the half-value of the mass thickness, for Emax = 2.27 MeV beta rays from the decay of Sr-90 in polyethylene?
How do those two values compare? How much more material is required to stop 50% of the gamma rays compared to 50% of the beta rays.
Which mass thickness of lead do you need to stop attenuate 99% of the gamma rays? Given the density of lead, 11.3 g/cm3, what is the thickness of such a sheet of lead?
Add any comments or questions you may have.