

Like all EM waves, light transports energy across space. The intensity (energy per unit area and unit time) is proportional to the square of the amplitude of the electric field of the light wave. This energy, however, arrives at a receiver not continuously but in discrete units called photons. The energy transported by an electromagnetic wave is not continuously distributed over the wave front. It is transported in discrete packages. Photons are the particles of light.
Problem:
Solution:
E = hc/λ.
Blue light: E
= (6.626*10-34 Js)(3*108 m/s)/(450*10-9 m)
= 4.4*10-19 J
= 2.76 eV
Red light: E
= (6.626*10-34 Js)(3*108 m/s)/(700*10-9 m)
= 2.8*10-19 J
= 1.8 eV
Problem:
E = hc/λ = (6.626*10-34 Js)(3*108 m/s)/(550*10-9 m) = 3.61*10-19 J = 2.26 eV.
To find the number of photons hitting the pages of a book, we need to know the energy per second that falls on the pages. Let us assume that the light energy from the bulb travels uniformly in all directions. Imagine a sphere centered on the light bulb with a radius R = 2 m. Every second, 15 J or so of visible light energy crosses this sphere. If the light energy is spread uniformly over the sphere, then the intensity of the visible light or the energy per unit area per unit time in units of W/m2 at any point on the inner surface of the sphere will be
I = Plamp/(4πR2),
where Plamp is the power of the visible light emitted by the lamp (~15 W) and 4πR2 is the area of the inner surface of the sphere.
I = (15/(16π))(W/m2)
The book pages essentially lie on the inner surface of this imaginary sphere, they have area A. The energy per unit time that falls on on this area is
Pbook = IA.
(Energy per unit time falling on area A = energy per unit time per unit
area times area A.)
Taking a typical hardcover book and measuring it with a ruler, we find that the two facing pages form a rectangle about 18 cm tall and 24 cm wide, so A = (0.18 m)(0.24 m) = 0.058 m2. So
Pbook = (15/(16π))*0.058 W = 0.017 J/s.
Finally, to find the number of photons per second falling on these pages, we have to divide Pbook by the energy per photon.
Number of visible photons from bulb hitting the book per second
= (0.017 J/s)/(3.61*10-19 J) = 4.7*1016/s.
This is not an accurate number, but a useful, order of magnitude estimate.
In 1905, Einstein used the discrete nature of light to explain the photoelectric effect. To demonstrate this effect light is shone on a metal surface. If the frequency of the light is higher than the cutoff frequency fc, then electrons are released. No photoelectric electrons are emitted if the frequency of the light falls below this cutoff frequency fc. For many metal surfaces the frequency of blue light is greater than fc and the frequency of red light is less than fc. If red light is shone on the surface, no electrons are emitted, no matter what the intensity of the light. If blue light is shone on the surface, electrons are emitted. The number of emitted electrons depends on the intensity of the light. But even if the intensity is reduced to a very low value, electrons are still emitted, albeit at a very low rate.
The photoelectric effect cannot be understood within the wave picture of light. To eject an electron from a metal surface a certain amount of energy Φ, called the work function of the metal, must be supplied to this electron. In the wave picture the energy of the light beam does not depend on the frequency, but only on the intensity, which is proportional to the square of the amplitude. Einstein explained the photoelectric effect by postulating that an electron can only receive the large amount of energy necessary to escape the metal from the EM wave by absorbing a single photon. If this photon has enough energy, the electron is freed. Excess energy appears as kinetic energy of the electron. The maximum kinetic energy of the electron is given by E = hf - Φ. If the photon does not have enough energy, then the electron cannot escape the metal.
Link:
Problems:
(a) What is the work function of the surface?
(b) What is the cutoff frequency of the surface?
Link:
The Compton effect or Compton scattering (discovered in 1923, Nobel Prize in Physics in 1927) is another famous experiment supporting the existence of photons. To observe the Compton effect, x-rays are scattered from (nearly) free electrons. To understand Compton scattering we must think of photons as particles.
Imagine an incoming photon scattering off a stationary electron.
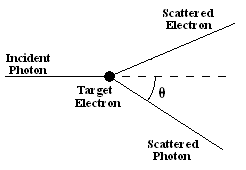
After the collision the photon's and the electron's trajectory will be at some angles with respect to the direction of the incoming photon. If we know the energy and momentum of the incoming photon, and the angle the outgoing photon's trajectory makes with the direction of the incoming photon, then we can determine the outgoing photon's energy using energy and momentum conservation.
The energy of a photon is E = hf = hc/λ and the
momentum of the photon is p = hf/c = h/λ = E/c.
The relativistically correct expression for the energy of the electron is Ee
= (pe2c2 + me2c4)1/2.
The initial momentum of the electron is (nearly) zero.
Energy conservation requires Ei + Eei = Ef + Eef,
or Ei + mec2 = Ef + (pef2c2
+ me2c4)1/2.
Momentum conservation requires pi = pf +
pef. This is a vector equation.
Rewriting these equations we have
(i) (pef2c2 + me2c4)1/2
= Ei + mec2 - Ef,
(ii) pef2 = pef•pef
= (pi - pf)•(pi
- pf) = pi2 + pf2
- 2pi•pf = pi2
+ pf2 - 2pipfcosθ,
where θ is the photon scattering angle.
Substituting (ii) into (i) and squaring we find
pi2 + pf2 - 2pipfcosθ
+ me2c2 = (Ei/c + mec - Ef/c)2
= (pi + mec - pf)2
= pi2 + pf2 - 2pipf
+ me2c2 + 2(pi - pf)mec,
or
pipf(1 - cosθ ) = (pi
- pf)mec,
(1 - cosθ)/(mec) = 1/pf - 1/pi.
It is traditional to rewrite this expression in terms of the change of the
photon's wavelength.
Δλ = (λf -
λi) = [h/(mec)](1 - cosθ).
The quantity h/(mec) = 0.00243 nm = 2.43 pm is called the Compton wavelength for photon-electron collisions. The Compton wavelength is the amount by which the photon's wavelength changes when it scatters through 90°. The change in wavelength is smaller if the scattering angle is less than 90°, and is a maximum of twice the Compton wavelength if the photon scatters backwards through 180°. The Compton wavelength for photon-electron collisions is small (~10-12 m) and the Compton effect is only observable with very short wavelength photons. Only then does the change in the wavelength Δλ become an appreciable fraction of the wavelength λ.
Problem:
Photons of wavelength λ scatter elastically on free protons initially at rest. By how much does the wavelength of the photons scattered at 90o increase?
For photon-proton collisions the Compton wavelength is even smaller small (~10-15 m) and the Compton effect is harder to measure and verify experimentally.