

|
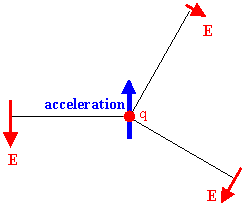
The particles that make up an object can have ordered energy and disordered energy. The kinetic energy of an object due to its motion with velocity v with respect to an observer is an example of ordered energy. The kinetic energy of individual atoms, when they are randomly vibrating about their equilibrium position, is an example of disordered energy. Thermal energy is disordered energy. The temperature is a measure of this internal, disordered energy. The absolute temperature of any substance is proportional to the average kinetic energy associated with the random motion of the substance. The velocity of particles with thermal energy is changing almost all the time. The particles are accelerating. The atoms and molecules are themselves complicated arrangements of charged particles. Accelerating charged particles produce electromagnetic radiation. The power radiated is proportional to the square of the acceleration. Higher rates of velocity change result in higher frequency (shorter wavelength) radiation. We therefore expect that atoms and molecules with thermal energy will emit radiation.
|
 |
How do you measure the intensity of radiation emitted by an object as a function of wavelength for a fixed temperature?
Problems:
Solution:
Use a blackbody. A blackbody is a body that absorbs all the radiation that falls onto it. It does not reflect any radiation. It reaches thermal equilibrium with its surroundings, and in thermal equilibrium emits exactly as much radiation it absorbs. It has emissivity = 1. Emissivity measures the fraction of radiative energy that is absorbed by the body.
Experimental realization:
A small hole in the side of a large box (oven) held at a constant temperature T. Any radiation that enters through the hole bounces around inside and has little chance of ever getting out again. Eventually it gets absorbed. Radiation coming out the hole is as good a representation of the radiation from a perfect emitter.
What is observed?
The observed intensity of the radiation emitted as a function of wavelength can be described by the Planck Radiation Law.
The primary law governing radiation is the Planck Radiation Law, which gives the intensity of radiation emitted by a blackbody as a function of wavelength for a fixed temperature. The Planck law gives a distribution, which peaks at some wavelength. The peak shifts to shorter wavelengths for higher temperatures, and the area under the curve grows rapidly with increasing temperature. The diagram below shows the intensity distribution predicted by the Plank law in J/(m2s) for blackbodies at various temperature.
|
|
In 1900 Max Planck presented a theoretical analysis of this distribution. He modeled the oscillating charges that must exist in the oven walls, radiating EM waves, and, in thermodynamic equilibrium, themselves being driven by the radiation field. He had to make a non-classical assumption. The oscillators can only lose or gain energy in discrete amounts, called quanta. If an oscillator has frequency f it gains or looses energy in quanta of size hf. The constant h is now called Planck’s constant, h = 6.626 * 10-34 Js. No-one, including Planck himself, realized the importance of this " clever technical fix" until, in March 1905, Albert Einstein turned his attention to the problem.
|
The Wien law and the Stefan-Boltzmann Law can be derived from the the Planck Radiation Law.
The Wien Law gives the wavelength of the peak of the radiation distribution, λmax = 3*106/T. Here λ is measured in units of nanometer = 10-9 m and T is in Kelvin.
The Stefan-Boltzmann Law gives the total energy being emitted at all wavelengths by the body.
Radiated power = emissivity * σ * T4 * Area
Here σ is the
Stefan-Boltzmann constant,
σ = 5.67*10-8 W/(m2K4).
The Wien law explains the shift of the peak to shorter wavelengths as the temperature increases, while the Stefan-Boltzmann law explains the growth in the height of the curve as the temperature increases. This growth is very abrupt, since it varies as the fourth power of the temperature.
Links: