

In-class group activity 5
See a demonstration of electron diffraction.
Open a Microsoft Word document to keep a log of your results and conclusions.
How does matter behave on a scale of a few nanometers or smaller? Is its behavior governed by Newton's laws or by a wave equation? The de Broglie relations associate a wavelength λ = h/p = h/√(2mE) with each particle of momentum p. For an electron which has been accelerated through a potential difference of 5 kV and therefore has a kinetic energy of 5000 eV = 8*10-16 J, this wavelength is λ = 1.74*10-11 m. Can we measure λ?
If a beam of accelerated electrons passes through a thin crystal, the crystal planes can act like a diffraction grating. (For a grating d sinθ = mλ.) Different crystal planes can produce different diffraction patterns. Planes that are spaced farther apart produce a narrower patterns. You will observe the diffraction pattern produced when 5 keV electrons pass through graphite.
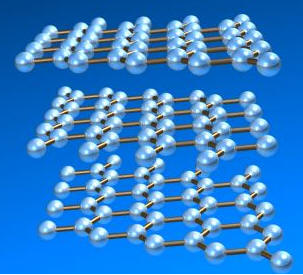
Graphite has the crystal structure shown below.
 |
 |
 |
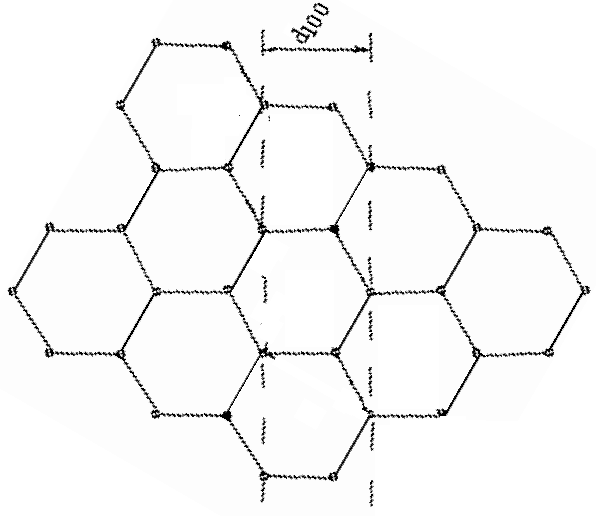
| In this orientation, the d100 planes produce a horizontal pattern. d100 = 2.10*10-10 m |
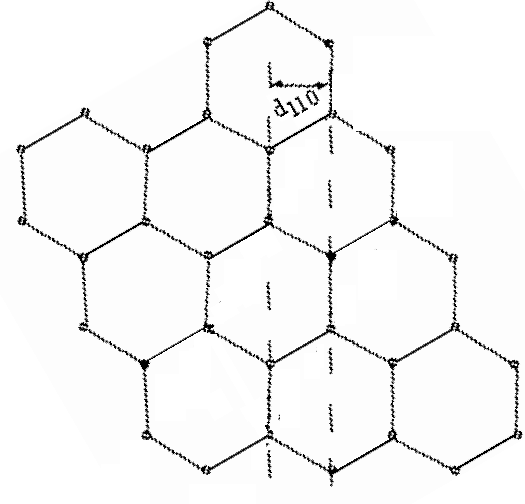
In this orientation, the d110 planes produce a horizontal pattern. d110 = 1.21*10-10 m |
The graphite in our apparatus is not a single crystal, but a polycrystalline powder. The orientation of the various crystals is random. Powder diffraction produces a pattern of concentric rings. The powder diffraction patters is just the superposition of the patterns produced by the individual crystals with random orientations.
| Assume for a single crystal with a fixed orientation we observe the pattern shown on the right. The diffraction pattern is rotated by the same angle as the crystal. |
 |
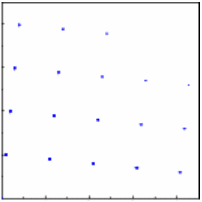 |
| Then for 4 crystals with 4 different orientations, we also observe 4 different orientations of the diffraction patterns. The individual diffraction patterns plotted in the same color as the corresponding crystal start to add up to rings. |
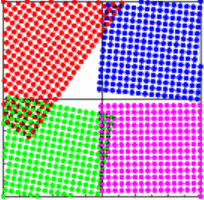 |
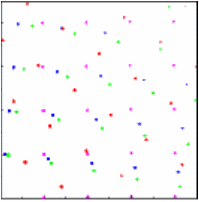 |
| For 40 randomly oriented crystals, powder rings become clearly visible. |
 |
|
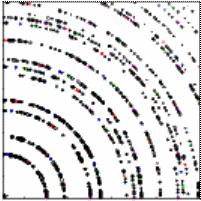
| In our experiment accelerated
electron with 5 keV kinetic energy pass through a graphite target in an
evacuated tube and hit a fluorescent screen. We observe the ring
pattern on this screen.
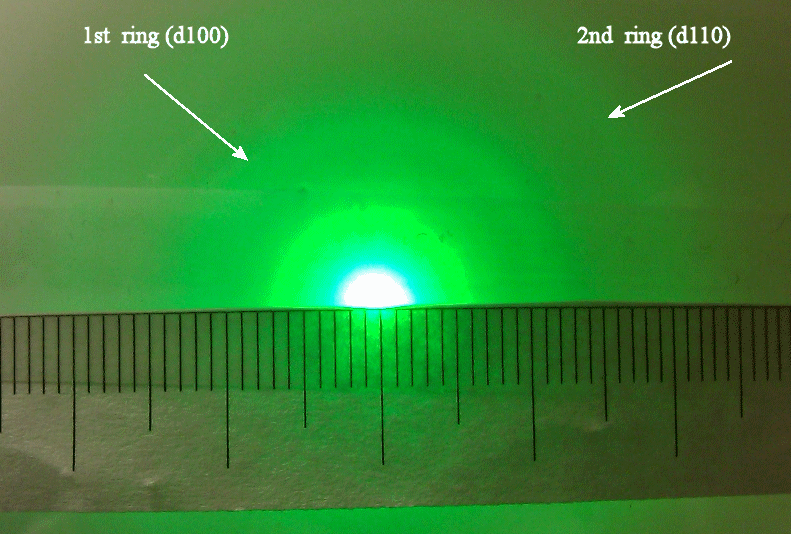
Two rings are clearly visible. These correspond to the first-order maxima produced by the d100 and the d110 planes. All other rings are too dim or at angles too large to observe with our apparatus. You will measure the diameter of the two visible rings and calculate the angles θ, given the distance L = 13.5 cm from the target to the screen. Using the known plane spacings d100 = 2.10*10-10 m and d110 = 1.21*10-10 m you will then use d sinθ = λ to experimentally determine the de Broglie wavelength λ of the 5 keV electrons. |
|
||||||||||||||||||
|
Schematic sketch off the
apparatus:
|
|
||||||||||||||||||
| Make the measurements and fill in
the table below. Measure the center-to-center distance for each
bright ring. The scale on the picture is a mm scale.
Discuss your results.
|
 |