

Solutions to the Schroedinger equation for a free particle of mass m, (-ħ2/(2m))∂2ψ(x,t)/∂x2 = iħ∂ψ(x,t)/∂t, are the plane waves ψ(x,t) = exp(i(kx - ωt)), and wave packets build from those plane waves. |ψ(x,t)|2 represents the probability per unit length of finding the particle at a time t at position x.
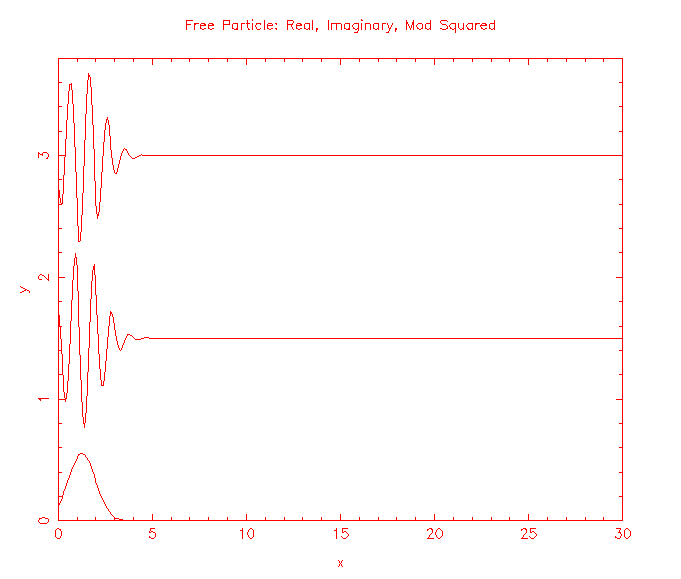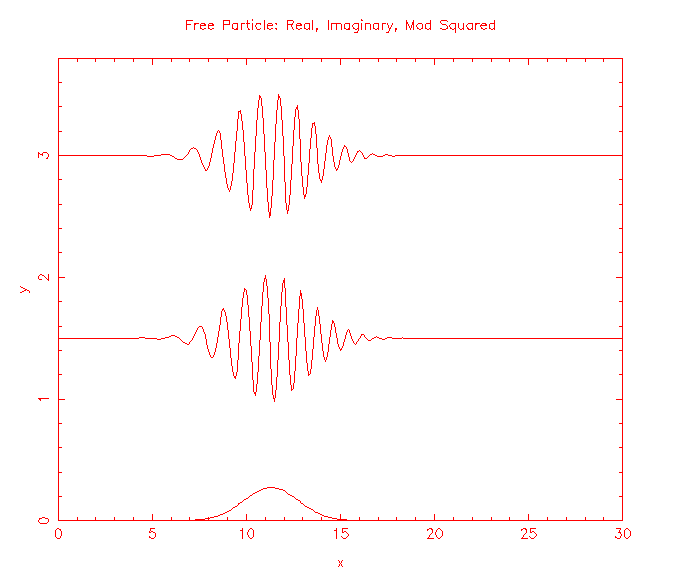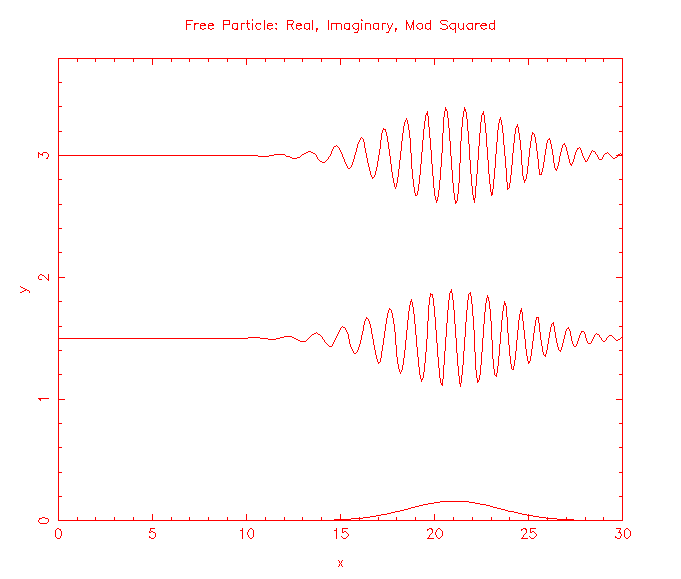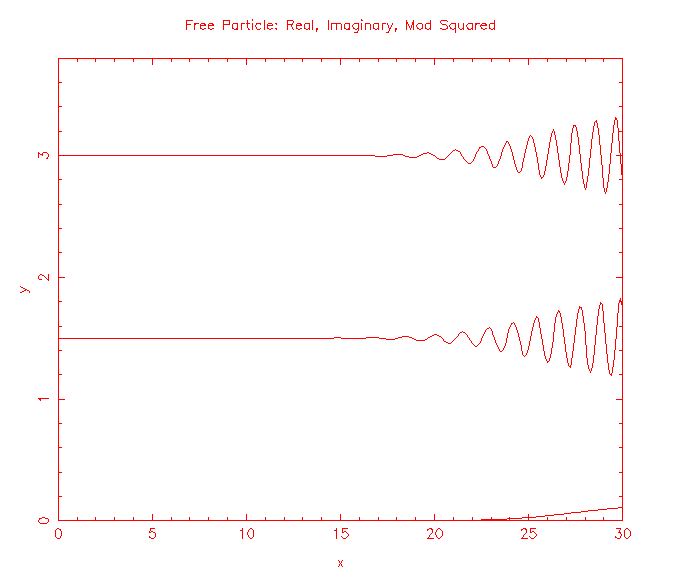
How do we make predictions about a particle that is not free, but is confined?
Stationary states
The Schroedinger equation for a particle moving in one dimension through a region where its potential energy is a function of position has the form
(-ħ2/(2m))∂2ψ(x,t)/∂x2 + U(x)ψ(x,t) = iħ∂ψ(x,t)/∂t.
We are often interested in finding the eigenstates of the energy operator iħ∂/∂t, i.e. we are interested in finding the wave functions of particles whose energy can be predicted with certainty. For an eigenfunction of the energy operator we have
iħ∂ψ(x,t)/∂t = Eψ(x,t), or ∂ψ(x,t)/∂t = (-iE/ħ)ψ(x,t).
To within a constant the function ψ(x,t) is equal to
its own derivative with respect to t. The exponential function behaves this way.
Recall that d(exp(at))/dt = a exp(at). Also recall that we
are taking a partial derivative with respect to t, and, as far as this derivative
is concerned, x is treated like a constant.
Therefore the solution to the above equation is
ψ(x,t) = ψ(x) exp(-iEt/ħ).
For eigenfunctions of the energy operator iħ∂/∂t the Schroedinger equation becomes time independent.
(-ħ2/(2m))∂2ψ(x)/∂x2 + U(x)ψ(x) = Eψ(x).
The operator (-ħ2/(2m))∂2/∂x2 + U(x) is called the Hamiltonian operator H, and the time-independent Schroedinger equation is often abbreviated as
Hψ(x) = Eψ(x).
If we find the possible solutions ψ(x) of the time-independent Schroedinger equation for a particular potential energy function U(x), then we can obtain the corresponding wave functions ψ(x,t) by just multiplying ψ(x) by exp(-iEt/ħ), where E is the appropriate eigenvalue for each solution.
The wave functions of particles whose energy can be predicted with certainty are of the form ψ(x,t) = ψ(x) exp(-iEt/ħ).
The probability density is
|ψ(x,t)|2 = ψ(x) exp(-iEt/ħ) ψ*(x) exp(iEt/ħ) = ψ(x)ψ*(x) = |ψ(x)|2 .
The probability of finding the particle at a particular position x for a particle with well defined energy is independent of time. It does not change with time. The wave functions of particles with well defined energy are therefore often called stationary states.
Energy and position are incompatible measurements. Knowing the energy of a particle prevents us from knowing its position and tracking it. A stationary-state wave function contains no information about the position of the particles as a function of time. All we get is probabilities. We can certainly make a measurement and determine the position of a particle at a particular time, but then we loose the information about its energy.
The time-independent Schroedinger equation involves only real variables and can have real solutions. The complex part of the solution ψ(x,t) is then contained in the function exp(-iEt/ħ).
Assume we want to solve the Schroedinger equation (-ħ2/(2m))∂2ψ(x)/∂x2
+ U(x)ψ(x)
= Eψ(x).
Dividing both sides of the equation by -ħ2/(2m)
and defining k12 = 2mE/ħ2,
k0(x)2 = 2mU(x)/ħ2,
and k(x)2 = k12 - k0(x)2
we can simplify the notation.
(-ħ2/(2m))∂2ψ(x)/∂x2 + U(x)ψ(x) = Eψ(x).
∂2ψ(x)/∂x2 - (2mU(x)/ħ2)ψ(x) + (2mE/ħ2)ψ(x) = 0.
∂2ψ(x)/∂x2 - k0(x)2ψ(x)) + k12ψ(x) = 0.
∂2ψ(x)/∂x2
+ (k12 - k0(x)2)ψ(x) =
0,
or
∂2ψ(x)/∂x2
+ k(x)2ψ(x) = 0,
Let us solve this equation for the "infinite square well". We assume U(x) = 0 for x = 0 to L, and U(x) = infinite everywhere else. A particle cannot penetrate a region with infinite potential energy, there is no chance that we can find it there, and its wave function in that region is zero. We put the particle in a one-dimensional box, out of which it has no chance of escaping.
In the region from x = 0 to x = L the potential energy U(x) = 0. The particle can freely move inside the box. Therefore k0(x) = 0 and k(x)2 = k12. Possible wave functions for the particle must satisfy the equation
∂2ψ(x)/∂x2 + k2ψ(x) = 0,
and they must be zero at x = 0 and x = L, because the wave function must be continuous and it is zero outside the region from x = 0 to x = L.
Real solutions of the Schroedinger equation which are zero at x = 0 are ψ(x) = Asin(kx), since ∂2sin(kx)/∂x2 = -k2sin(kx) and sin(0) = 0. For these solutions to be zero at x = L we need
sin(kL) = 0, kL = nπ, with n = 1, 2, 3, ...,
since the sine function is zero if its argument is an integer multiple of π.
The possible values of k are kn = nπ/L, the possible values of the energy En = h2kn2/(2m) are En = n2π2ħ2/(2mL2). The potential and the first five possible energy levels a particle can occupy are shown in the figure below. Arbitrary units are used.
The energy of the particle in the infinite square well is
quantized. It can only take on the values En = n2π2ħ2/(2mL2).
If we measure the energy we can only measure one of these eigenvalues, En
= n2π2ħ2/(2mL2),
n = 1, 2, 3, ... . The confinement of the
particle leads to energy quantization.
If we measure En, then right after the measurement the wave function of
the particle is ψn(x, t)
= Ansin(nπx/L)exp(-iEnt/ħ).
For example if we measure the energy to be E3 = 9π2ħ2/(2mL2),
then right after the measurement the wave function of the particle is
ψ3(x,t)
= A3sin(3πx/L)exp(-iE3t/ħ).
A measurement changes the information an observer has about the system and therefore can change the wave function of the system.
The square of the normalized wave function |ψn(x,t)|2 = |ψn(x)|2 = An2sin2(nπx/L) is equal to the probability per unit length of finding the particle with energy En at position x. To normalize the wave function we have to choose An2 = 2/L. Then ∫-∞+∞|ψ(x,t)|2dx = 1, and the total probability of finding the particle inside the well is 1.
Example:
An electron (m = 9.109*10-31 kg) is confined in a one-dimensional infinite square well of width L = 10 nm. The figure below shows
(a) the lowest five energy levels En and the wave functions ψn(x) = (2/L)1/2sin(nπx/L)
(b) the corresponding probability density functions |ψn(x)|2 = (2/L)sin2(nπx/L)
The wave functions and the probability density functions have an arbitrary magnitude (i.e. they are not normalized) and are shifted by the corresponding electron energy
The lowest possible energy of the confined particle is its ground-state energy. All other possible energies are are excited-state energies. A particle can make transitions between different energy levels when it is interacting with its environment. If a particle makes a transition from a lower energy level to a higher energy level, it has to absorb an amount of energy ∆E = Ehigh - Elow from the outside world. It can absorb a photon with energy hf = ∆E, or it can receive the required energy from another particle in a collision. If the particle makes a transition from a higher energy level to a lower energy level, it has to release an amount of energy ∆E = Ehigh - Elow to the outside world. One way to do this is to emit a photon of energy hf = ∆E.
Because the energy levels are quantized, the energies of photons involved in transitions are quantized. The particle in the well can only interact with photons of certain energies, it can only emit or absorb light of certain colors.
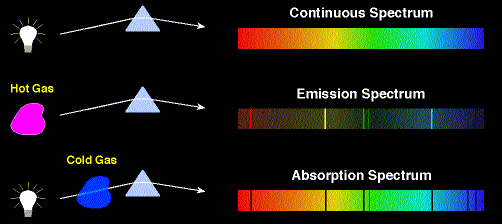
Another famous experiment that reveals energy quantization in atoms is the Franck–Hertz experiment.
Zero-point energy
In a quantum mechanical system such as the particle in the infinite square well, the ground-state energy is not zero. This lowest possible kinetic energy is called the zero-point energy. Classically a particle at rest in a well has zero kinetic energy and zero velocity. In quantum mechanics the uncertainty principle implies that if the velocity is measured to be exactly zero with certainty, the uncertainty in the position must be infinite. This violates the condition that the particle remain in the well. Quantum mechanics therefore dictates that the minimum velocity is never equal to zero, and hence the minimum kinetic energy is never equal to zero.
Problems:
An electron is confined to a 1 micron thin layer of silicon. Assuming that the semiconductor can be adequately described by a one-dimensional quantum well with infinite walls, calculate the lowest possible energy within the material in units of electron volt. If the energy is interpreted as the kinetic energy of the electron, what is the corresponding electron velocity? (The effective mass of electrons in silicon is m* = 0.26 m0, where m0 = 9.11 x 10-31 kg is the free electron rest mass).
Solution:
The lowest energy in a quantum well is
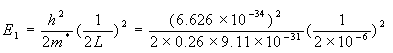
= 2.32 x 10-25 Joules = 1.45 meV.
The velocity of an electron with this energy equals:
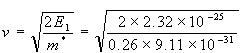
= 1.399 km/s.
A particle in a 1-D box has a minimum allowed energy of 2.5 eV.
(a) What is the next higher energy it can have? And the next higher after
that? Does it have a maximum allowed energy?
(b) If the particle is an electron, how wide is the box?
(c) The fact that particles in a 1-D box have a minimum energy is not completely
unrelated to the uncertainty principle. Find the minimum momentum of a
particle, with mass m, trapped in a 1-D box of size L. How does this
compare with the momentum uncertainty required by the uncertainty principle, if
we assume ∆x = L?
(a) En = n2π2ħ2/(2mL2)
E1 = 2.5 eV, E2 = 4*2.5 eV = 10 eV, E3
= 9*2.5 eV = 22.5 eV. There is no upper limit
(b) 2.5 eV*1.6*10-19 J/eV = π2(1.05*10-34
Js)2/(2*9.109*10-31 kg*L2)
L = 3.86*10-10 m.
The box has a width that is comparable to the typical size of an atom.
(c) E1 = pmin2/(2m), pmin2
= (2*9.109*10-31 kg*2.5 eV*1.6*10-19 J/eV),
pmin = 8.5*10-25 kg m/s.
∆p = ħ/∆x =
(1.05*10-34 Js)/(3.86*10-10 m) = 2.7*10-25
kg m/s. The minimum momentum required by the uncertainty principle is
approximately equal to ∆p.
∆p and pmin have the same order of
magnitude.
An electron is confined to a box of length 0.6 nm (a typical atomic size). From the uncertainty principle, estimate the minimum kinetic energy (in eV) of the electron.
The momentum must be of order
p =
ħ/L
= (1.05*10-34 Js)/(6*10-10 m).
One can now estimate the kinetic energy with
KE =
p2/(2m)
= p2/(2*9.109*10-31 kg)
= 1.68*10-20 J = 0.1 eV
Position and momentum
If we have measured the energy of a particle in an infinite square well, we know the energy exactly, but we cannot know its position or its momentum exactly. The energy, position and momentum of a particle in an infinite square well cannot be all measured simultaneously.
Given its energy, we can predict the average position of the particle in the well and also the probability that we will find it at a particular
position.
To find the average position we calculate <x> = ∑ xi |ψ(xi)|2∆xi.
The probability of finding the particle between x and x + ∆x
is |ψ(x)|2∆x .
To find the average value of any other observable whose operator is O, we have to calculate <O> = ∑ ψ(xi,t)*O ψ(xi,t)
∆xi.
This is the general rule for calculating average values of an observable in
quantum mechanics.
So to find the average value of the momentum we calculate <p> = ∑ψ(xi,t)*(ħ/i)(∂ψ(xi,t)/∂x)
∆xi.
Example:
For a particle in an infinite square well with L =1 unit let
ψ1(x)
= √2 sin(πx).
Open the linked spreadsheet.
Change ψ1(x) = √2 sin(πx) to ψ2(x) = √2 sin(2πx) and answer the same question.