Nuclear properties
Nuclei consist of positively charged protons and electrically neutral
neutrons packed closely together. Protons and neutrons are each about
1.4*10–15 m in diameter, and the size of a nucleus is
essentially the size of a ball of these particles.
The total number of nucleons in a nucleus is usually denoted by the mass
number A, where A = Z
+ N, Z protons and N neutrons. The chemical properties of
an atom are determined by the number of electrons, the same as the number of
protons Z. This is called the atomic number. Nuclei can have the same
atomic number, but different numbers of neutrons. These nuclei are called
isotopes.
The nuclear force which holds the nucleus
together is attractive, strong, and short ranged. Its range is about equal
to 4 proton diameters. The nuclear force treats
protons and neutrons equally and does not act on electrons.
Iron 56 has the largest binding energy per nucleon, no other nucleus is more
tightly bound. The reason Iron 56 is most strongly bound is because the diameter of an iron
nucleus is about equal to the range of the nuclear force. Iron 56 is the largest nucleus in which every
nucleon attracts every other nucleon. The diameter of the Iron 56 nucleus
is the distance over which the attractive nuclear force is stronger than the
repulsive electrostatic force for each particle pair.
Atomic and nuclear data tables often list the mass of the neutral atom (not
that of the nucleus) in atomic mass units (u).
In terms of the nuclear masses, we write for the
binding energy B(Z,N) of a
nucleus with Z protons and N neutrons
B(Z,N) = c2(Z*mp + N*mn - Mnuc(Z,N)).
In terms of the atomic masses, we write
B(Z,N) = c2(Z*mH + N*mn - Matom(Z,N)).
Nuclear decay
The decay of an unstable nucleus is a random (probabilistic) process.
The rate at which nuclei decay, dN/dt, is proportional to the number N of nuclei
present.
dN/dt = -λN, N(t) = N0exp(-λt).
Different radionuclides decay at different rates, each having its own
decay constant λ. Their mean
life is τ = 1/λ.
The half-life of a nucleus is given by t1/2 = τ ln2 = ln2/λ.
Alpha decay is a form of radioactive decay in which an
atomic nucleus characterized by mass number A and atomic number Z ejects an
alpha particle and transforms into a nucleus with mass number A - 4 and and atomic
number Z - 2 .
Example: 238U --> 234Th + α
Alpha decay is a quantum-mechanical tunneling process. The quickest
decays correspond to the most energetic
α-particles.
Beta decay is a type of
radioactive decay in which a β-particle
is emitted.
In β−
decay, the weak nuclear interaction converts a neutron into a proton
while emitting an electron and an anti-neutrino.
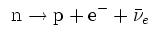
In β+ decay,
a proton is converted into a neutron, a positron and a neutrino.
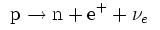
If the proton and neutron are part of an atomic nucleus
the decay processes transmute one chemical element into another.
γ-decay
is the emission of a photon by an exited state of a nucleus, as it returns to
its ground state.