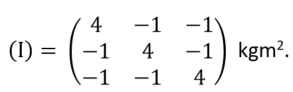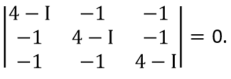Problem 1:
Consider a rectangular lamina with dimensions A and B, and mass M. The
lamina is attached to a wall with a nail placed at a point P = P(x,y), where the
coordinates x and y are referred to the center of the lamina.
(a) Determine the position of stable equilibrium.
(b) Describe the motion of the lamina when it is slightly displaced from this
equilibrium position.
Solution:
- Concepts:
Stable equilibrium
- Reasoning:
The potential energy U has a minimum at the position of stable equilibrium.
 Details of the calculation:
Details of the calculation:
(a) Potential energy: U = -Mgdcosθ = -Mg(x2 + y2)½cosθ.
Extreme U: dU/dθ = 0, sinθ = 0, θ = 0 or π.
θ = π, CM lies directly above the pivot point --> unstable equilibrium.
θ = 0, CM lies directly below the pivot point --> stable equilibrium.
(b) For small angles θ near θ = 0 we have U(θ) = U(0) + dU/dθ|0θ
+ ½ d2U/dθ2|0θ2 +
... .
d2U/dθ2 = Mgdcosθ. U(θ) = U(0) + ½Mgdθ2,
keeping only terms up to second order in θ.
U(θ) = U(0) + ½kθ2 with k = Mgd.
τ = I d2θ/dt2 = -dU/dθ = - Mgdθ. We have a restoring
torque.
To find I we use the parallel axis theorem.
ICM = (1/12)M(A2 + B2). I(x,y) = ICM
+ M(x2 + y2)= ICM + Md2 = M(A2/12
+ B2/12 + x2 + y2).
d2θ/dt2 = -g(x2 + y2)½(A2/12
+ B2/12 + x2 + y2)θ.
Frequency of oscillations: ω2 = g(x2 + y2)½(A2/12
+ B2/12 + x2 + y2).
Problem 2:
A cylinder with mass m, radius R, and uniform density starts rolling without
slipping from rest in a train that accelerates with constant acceleration a0.
The axis of the cylinder is perpendicular to the motion of the train as shown
below. Find the acceleration of the cylinder in the lab frame (rest frame) and
in the train frame.
Solution:
- Concepts:
Motion in an accelerating frame.
- Reasoning:
In an accelerating frame fictitious forces appear. The net force in such a
frame is
F = Finertial - ma, where a is the
acceleration of the frame.
- Details of the calculation:
Let the acceleration of the train point in the x-direction.
In the train frame, the net force on the ball is F = -ma0 + f = ma'
where f is the force of static friction preventing the contact point from
slipping and a' is the acceleration of the CM of the cylinder. Friction exerts
a torque on the cylinder, |τ| = fR = I|α| = I|a'|/R.
If the z-axis points up this torque points in the -y direction.
f = -Ia'/R2 since f points in the positive and a' in the negative
x-direction.
-ma0 = ma' + Ia'/R2. a' = -a0/(I/mR2
+ 1).
For the cylinder I = ½mR2, a' = -(2/3)a0 is the
acceleration of the cylinder in the train frame.
The acceleration of the cylinder in the frame of the stationary floor is a''
= a0 + a' = a0/3.
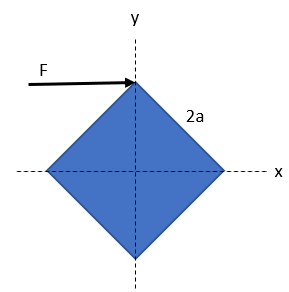
Problem 3:
A square plate with uniform density, mass m, and of side length 2a, lies on a
frictionless table as shown in the figure. A force F = F i
is
applied for a very short time Δt and delivers an impulse Δp to the upper
corner. Find the position of this corner as a function of time.
Solution:
- Concepts:
Δp = FΔt, ΔL =
τΔt
- Reasoning:
The same force provides the linear and angular impulse.
The force is pointing in the x-direction.
- Details of the calculation:
FΔt = mΔv = mv i, √2 a FΔt = ΔL = Iω.
L and ω point in the -z direction, the rotation is clockwise.
v i = i Δp/m = is the velocity of th CM and ω is
angular velocity about the CM after the impulse.
The moment of inertia of the
plate about an axis normal to the plate through its its CM is
I = 2ma2/3.
[I = 4∫0adx∫0ady ρ(x2
+ y2), with ρ = m/4.]
We therefore have
ω = √2 a mv/I = (3/√2) v/a.
The position of the corner as a function of time is
x(t) = vt + √2 a sin(ωt), y(t) = √2 a cos(ωt), or
x(t) = Δpt/m + √2 a sin(3Δpt/(am√2)),
y(t) = √2 a cos(3Δpt/(am√2)t).
Problem 4:
You have a system of 4 particles of equal mass M = 1 kg. One sits at the origin
(x, y, z) = (0, 0, 0) m. The others sit at (1, 1, 0), (0, 1, 1) m, (1, 0, 1)
m. These particles are attached to each other by massless rigid rods such that
they will always maintain their positions relative to one another.
(a) Calculate the full moment of inertia tensor for this system.
(b) Find the principal axes for this system.
In case it's useful to you, here's a fact:
(4 − x)3 − 3(4 − x) − 2 = (2 − x)(5 − x)2.
Solution:
- Concepts:
The inertia tensor
- Reasoning:
Iij = Σkmk[Σl(xk)l2δij
- (xk)i(xk)j] is the inertia tensor.
The Iii are called the moments and the Iij (i ≠ j) are
called the products of inertia.
- Details of the calculation:
(a)
Ixx = M Σk(yk2 + zk2)
= M(0 + 1 + 2 + 1) = 4 kgm2.
Iyy = M Σk(xk2 + zk2)
= M(0 + 1 + 1 + 1) = 4 kgm2.
Izz = M Σk(xk2 + yk2)
= M(0 + 2 + 2 + 1) = 4 kgm2.
Ixy = Iyx = -MΣkxkyk =
-M(0 + 1 + 0 + 0) = -1 kgm2.
Iyz = Izy = -MΣkzkyk =
-M(0 + 0 + 1 + 0) = -1 kgm2.
Ixz = Izx = -MΣkxkzk =
-M(0 + 0 + 0 + 1) = -1 kgm2.The moment of inertia tensor is
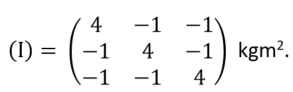
(b) Li = ΣjIijΩj. If Ω
points along a principal axis then L and Ω
are aligned and Li = IΩi, where I is
one of the principal moments of inertia.
Then Σj(Iij - I δij)Ωj = 0, |Iij
- I δij|= 0.
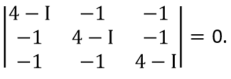
(4 − I)3 − 3(4 − I) − 2 = (2 − I)(5 − I)2 = 0.
I1 = 2, I2 = I3 = 5, we have a symmetric top.
To find the corresponding principal axis we use:
I1 = 2:
2a1 - a2 - a3
= 0, -a1 + 2a2 - a3 = 0, --> a1 = a2
= a3, the principal axis vector is (1, 1, 1).
I2 = I3 = 5:
-a1 - a2 - a3
= 0.
This equation defines a plane perpendicular to (1, 1, 1).
We can choose any
orthogonal pair of axes in that plane, for example (1, -1, 0) and (1, 1, -2).
 Details of the calculation:
Details of the calculation: