Find the rotational energy levels of a diatomic molecule with atoms of mass m1 and m2. Take r1 and r2 to be the distances from atoms 1 and 2 to the center of mass.
(a) Given that the radial function for the ground state of hydrogen is R(r) = (2/a03/2) exp(-r/a0),
find the value of radius at which the ground state radial probability
reaches a maximum.
(b) What is the probability of finding the ground state electron within the Bohr
radius?
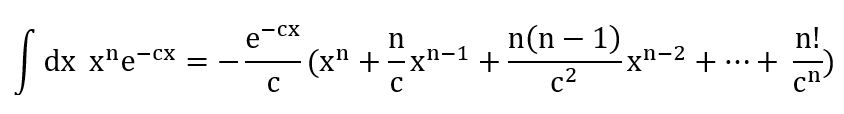
(c) Using your calculation in parts (a) and (b), comment on the similarity and the difference of the hydrogen atom ground state in quantum mechanics compared to the Bohr model of the hydrogen atom (in terms of energy and radius), and give a short explanation of how the quantum mechanical model solves the uncertainty issue in the Bohr model.
A particle with mass m is in ground state of 1D harmonic oscillator potential. The
potential energy function is U(x) = ½mω2x2.
(a) A position measurement is made. What is the probability of
finding the particle at the middle of the potential well with a position
uncertainty
Δx << (ħ/(mω))½?
(b) If the particle is found within
Δx at the middle of the potential well, what is the probability that a
subsequent energy measurement will still find the particle in the ground state?
A negative K meson with mass m = 1000 electron masses is captured into a circular
Bohr orbit around a lead nucleus (Z = 82). Assume it starts with principal quantum
number n = 10 and then cascades down through n = 9, 8, 7, ... etc.
(a) What is the energy of the photon emitted in the n = 10 to n = 9 transition?
(b) What is the approximate radius of the lead nucleus if no further quanta are
observed after the n = 4 to n = 3 transition (because of nuclear
absorption of the K meson)?