

For the purpose of this problem, consider the m- to be a heavy electron.
(a) Find the normalized wave function for a m-
- 3He++ bound system in its ground state.
(The system consists of a m- orbiting a 3He++
nucleus.)
(b) What is the ground state binding energy of this system in terms of the ground state binding energy of the hydrogen atom?
(c) Compare <r> and <r2> for the ground state of the m- - 3He++ system and for the hydrogen atom.
(d) If a nucleus consisting of A-Z neutrons and Z protons suddenly decayed into a nucleus of A-Z-1 neutrons and Z+1 protons, how would you find the probability that the electron will remain in the ground state in terms of |y100>before and |y100>after?
Given: mp=938 MeV/c2, me=0.5 MeV/c2, mm=207 me.
Find the wave functions and energy levels of the three-dimensional isotropic harmonic oscillator
(a) in spherical coordinates, and
(b) in Cartesian coordinates.
(c) Express the wave functions nr=1, l=1, in spherical
coordinates as a linear combination of the wave functions obtained in Cartesian
coordinates.
The wave function of a stationary state of a hydrogen atom is
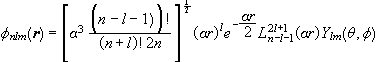 ,
,
where ![]() .
.
The Laguerre polynomials are normalized in the following way:
![]() .
.
Show that for any stationary state of a hydrogen atom the mean value of the kinetic energy is equal in magnitude and opposite in sign to the eigenvalue of the energy, i.e. En=-<p2/2m>nlm.
Note: H=p2/2m-e2/r.
For hydrogen-like atoms, such as the alkali atoms, the screening effect of the "closed-shell" electrons can be accounted for by considering the electron to move in the potential V(r)=-(e2/r)(1+a/r), where a is a constant. Find the energy eigenvalues for this potential.