

The ground state wave function for a hydrogen like atom is
![]() ,
,
where a0 = ħ2/(μe2)
and μ is the reduced mass, μ ~
me = mass of the electron.
(a) What is the ground state wave function of tritium?
(b) What is the ground state wave function of
3He+?
(c) An electron is in the ground state of tritium. A nuclear reaction
instantaneously changes the nucleus to 3He+. Assume the beta particle and the
neutrino are immediately removed from the
system. Calculate the probability that the electron remains in the ground
state of 3He+.
Solution:
Details of the calculation:
(a) For a hydrogen atom we have
![]() .
.
Defining
![]() ,
,
we write
![]() .
.
For a hydrogenic atom we have
![]() .
.
![]() .
.
Defining
![]() ,
,
we write
![]() .
.
The same equations have the same solutions. To find the eigenfunctions and eigenvalues of the Hamiltonian of a hydrogenic atom we therefore replace in
the eigenfunctions of the Hamiltonian of the hydrogen atom
![]() , and in the eigenvalues of the Hamiltonian of the hydrogen atom we replace
, and in the eigenvalues of the Hamiltonian of the hydrogen atom we replace
![]() .
.
(a) Tritium: The reduced mass
is![]() ,
,
assuming the neutron mass equals the
proton mass.
The nuclear charge is Z = 1. Since a0' ~ a0 the ground state
wave function of the
tritium is the ground state wave function of the hydrogen atom.
![]() .
.
(b)
3He+: The reduced
mass is
![]() ,
,
assuming the neutron mass equals
the proton mass.
The nuclear charge is Z = 2. Since a0' ~ a0/2 the ground state
wave function of the
3He+ is
![]() .
.
(c) Sudden approximation:
![]()
![]() .
.
![]() .
.
A negative K meson with mass m=1000 electron masses is captured into a circular Bohr orbit around a lead nucleus (Z=82). Assume it starts with principal quantum number n=10 and then cascades down through n=9,8,7,... etc.
(a) What is the energy of the photon emitted in
the n=10 to n=9 transition?
(b) What is the approximate radius of the lead nucleus if no further quanta are
observed after the n=4 to n=3 transition (because of nuclear absorption of
the K meson)?
We have a hydrogen like system with Z=82:
![]() .
.
![]() .
.
![]() .
.
This is the approximate radius of the lead nucleus.
![]() ,
,
for Z=1.
An electron in the n = 2, l = 1, m = 0 state of
a hydrogen atom has a wave function ψ(r,θ,φ)
proportional to r exp(-r/(2a0)) cosθ, where a0
is the Bohr radius.
(a) Find the normalized wave function
ψ(r,θ,φ).
(b) Find the probability per unit length of finding the electron a distance
r from the nucleus.
(c) Find the most probable distance RMP of the electron from
the nucleus.
(d) Find the average distance <r> from the nucleus.
Solution:
Details of the calculation:
(a) Normalization:
![]() .
.
![]() .
.
![]() .
.
![]() .
.
![]() .
.
(b) P(r,θ,φ)d3r = |ψ(r,θ,φ)|2d3r is the probability of finding the electron in an infinitesimal volume d3r about r = (r,θ,φ). P(r,θ,φ) is the probability per unit volume.
![]()
P(r) is the probability per unit length.
![]() .
.
(c) We are looking for the maximum in P(r).
![]()
(d) ![]() 0.
0.
Problem:
Find the momentum space wave function Φ(p) for the electron in the 1s state of the hydrogen atom.
Solution:
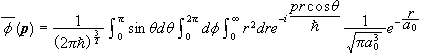

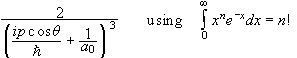
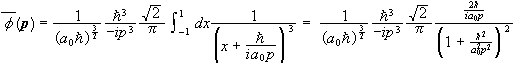 .
.
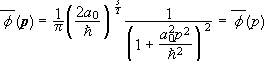 .
.