
The hydrogen atom consists of a proton of mass mp=1.7´10-27kg and charge qe=1.6´10-19C and an electron of mass me=9.1´10-31kg and charge -qe. The dominant part of the interaction between the two particles is the electrostatic interaction. The electrostatic potential energy is
![]()
in SI units.
If we confine ourselves to studying the relative motion of the two particles, and if we neglect any external forces, and if we treat the particles as spinless particles, then the Hamiltonian of the system is
 .
.
The reduced mass m of the system is nearly the same as the electron mass me, and the center of mass of the system is nearly in the same place as the proton. We therefore often call the relative particle "the electron" and the center of mass "the proton".
![]() .
.
H is the Hamiltonian of a fictitious particle moving in a central potential. We already know that the eigenfunctions of H are of the form
![]() ,
,
where ukl(r) satisfies the radial equation
![]()
with the condition ukl(0)=0.
We define the effective potential ![]() .
.
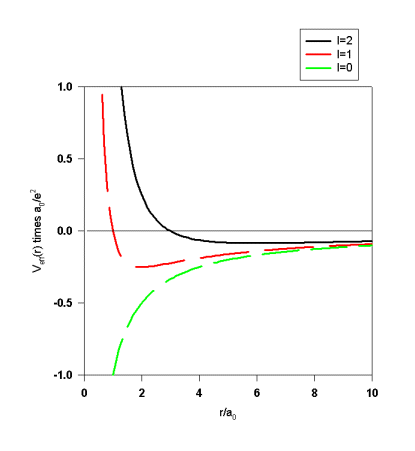
In terms of the effective potential the radial equation becomes
![]() .
.
As r approaches infinity, Veff(r) goes to zero. For every Ekl>0 there therefore exists a solution ukl(r) with an oscillatory behavior at infinity. These solutions represent unbound states.
For Ekl<0 ukl(r) will be proportional to
![]() as r approaches infinity.
The real
positive exponential solution is physically unacceptable. As r goes to zero, ukl(r)
becomes proportional to rl+1. Only for certain discrete values of Ekl
is it possible to properly match these two asymptotic solutions.
as r approaches infinity.
The real
positive exponential solution is physically unacceptable. As r goes to zero, ukl(r)
becomes proportional to rl+1. Only for certain discrete values of Ekl
is it possible to properly match these two asymptotic solutions.
In order to simplify the radial equation, we introduce the dimensionless quantity
![]() , where
, where ![]() .
The radial equation then becomes
.
The radial equation then becomes
![]() .
.
We want to solve this equation for Ekl<0 to find the energies of the bound states of the hydrogen atom.
Defining
![]()
we have
![]() .
.
We therefore try a solution of the form
![]() .
.
c
kl satisfies the equation![]() .
.
We have
![]() .
.
Therefore
![]() .
.
We may write this as ![]() .
.
The coefficients aq for each power q of r
must vanish for this to hold for all r. For
the power q+l-1 we therefore must have ![]() .
.
![]() .
.
We have found a recurrence relation for the coefficients cq. Fixing c0 allows us to calculate all cq.
We have
![]() .
.
As ![]()
![]() .
.
This implies that as ![]() ckl(r) becomes proportional
to
ckl(r) becomes proportional
to ![]() , since
, since
![]() .
.
The asymptotic behavior of ukl(r) is then
dominated by ![]() which is physically
unacceptable.
which is physically
unacceptable.
In order to keep the wavefunction finite at infinity the power series defining ![]() must terminate.
For some integer q=k we need ck=0. We then have ck(q>k)=0. For ck=0 we need
must terminate.
For some integer q=k we need ck=0. We then have ck(q>k)=0. For ck=0 we need ![]() .
.
Since we require c0 to be non zero, the possible values for k
are k=1, 2, 3, × × ×. For a given l the
possible values of Ekl then are ![]() .
.
c
kl(r) is a polynomial whose lowest order term is rl+1and whose highest order term is rk+l.In terms of c0 the various coefficients cq are calculated using the recurrence relations
![]() .
.
![]() .
.
![]() .
.
The associate Laguerre polynomials are defined as
![]() .
.
We therefore have
![]() .
.
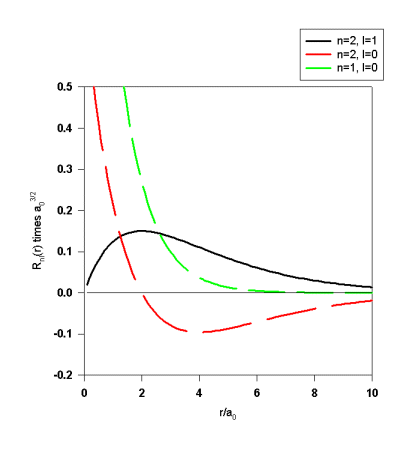
ckl(r) is proportional to a polynomial of order k-1 and therefore has k-1 radial nodes.
![]() .
.
![]() . Normalization requires
. Normalization requires ![]() .
.
![]() .
.
We therefore have
![]() .
.
![]() .
.
For each l there exists an infinite number of possible energies, corresponding to values of k=1, 2, 3, × × ×. Each of them is at least 2l+1 fold degenerate. This is the essential degeneracy, since Ekl does not depend on m. Accidental degeneracies also exist. For the hydrogen atom we find that Ekl is only a function of k+l, and we define k+l=n for the hydrogen atom.
The possible eigenenergies therefore are ![]() .
Here n is called the principal quantum
number, n fixes the energy of the eigenstate. Given n, l
can take on n possible values
.
Here n is called the principal quantum
number, n fixes the energy of the eigenstate. Given n, l
can take on n possible values ![]() . n
characterizes an electron shell, which contains n
subshells characterized by l. Each subshell
contains 2l+1 distinct states.
. n
characterizes an electron shell, which contains n
subshells characterized by l. Each subshell
contains 2l+1 distinct states.
The total degeneracy of the energy level En is ![]() for a hydrogen atom made from spinless
particles.
for a hydrogen atom made from spinless
particles.
The ground state energy of the hydrogen atom is -EI.
![]()
where
![]() .
.
a is the fine structure constant.
![]() .
.
We also have
![]() .
.
RH is the Rydberg constant.
![]() .
.
We denote the energy eigenstates of the hydrogen atom by { |n,l,m> }, where n=k+l.
The corresponding eigenfunctions are usually written as ![]() .
.
![]() , where k=n-l, and ukl(r) is given above. The number of radial nodes of Rnl(r)
is n-l-1.
, where k=n-l, and ukl(r) is given above. The number of radial nodes of Rnl(r)
is n-l-1.
Letters of the alphabet are associated with various values of l.
| l=0 | s |
| l=1 | p |
| l=2 | d |
| l=3 | f |
| l=4 | g |
Continue in alphabetic order.
The subshells are denoted by:
| n | l | subshell |
| n=1 | l=0 | 1s |
| n=2 | l=0 | 2s |
| n=2 | l=1 | 2p |
etc.
The principal shells are denoted by:
| n | principal shell |
| n=1 | K-shell |
| n=2 | L-shell |
| n=3 | M-shell |
Continue in alphabetic order.
Problem:
In the interstellar medium electrons may recombine with protons to form
hydrogen atoms with high principal quantum numbers. A transition between
successive values of n gives rise to a recombination line.
(a) A radio recombination line occurs at 5.425978 *
1010 Hz for a n = 50 to n = 49 transition. Calculate the Rydberg constant for H.
(b) Compute the frequency of the H recombination line corresponding to
the transition n = 100 to n = 99.
(c) Assume the mean speed in part (b) is 106 m/s. At what
frequency or frequencies would the recombination line be observable?
(d) Consider that radio recombination lines may be observed at either of two
facilities, the 11 meter telescope at Kitt Peak near Tuscon, Arizona, and the
1.2 meter radio telescope at Columbia University in New York. Relative larger
blocks of time are available on the smaller telescope, but its intrinsic noise
is moderately high. Where would you choose to map recombination radiation
emanating from an external galaxy. Discuss both technical and non technical
aspects of your choice.
Solution:
Details of the calculation:
(a) En' - En
= hν = hcRH(1/n2 -
1/n'2).
5.425978 *
1010 = 2.99792458 *
108RH(1/492 - 1/502), RH
= 1.097373 *
107 m-1.
(b) ν = cRH(1/992
- 1/1002) = 6.679710 *
109 s-1.
(c) The line is Doppler shifted.
ν'
= ν[(1
+ v/c)/(1 - v/c)]1/2 ~ ν(1
+ v/c) if v/c << 1.
Here v is the relative velocity of source and observer; v is positive
if the source and the observer approach each other, and v is negative if the
source and the receiver recede from each other.
-106/(3*108) < v/c < 106/(3*108),
6.657 *
109 s-1 < ν' < 6.702 *
109 s-1.
(d) Here are some factors worth considering:
The amount of radiation gathered by a telescope is proportional to the
square of its diameter D.
The smallest angle that can be resolved is approximately θ = λ/D.
(For each photon we have ΔxΔpx
~ h, Δx ~ D, ΔPx
~ h/D, θ = Δpx/p
= hc/(Dhν) = λ/D.)
If you assume that the radiation has a frequency of ~ 10-10 s-1, then
λ = c/ν = 3 cm. For the small telescope we therefore
have θ = 0.03/1.2 while for
the large telescope we have θ = 0.03/11. It takes
(11/1.2)2 = 84 times as long to gather
the same amount of radiation with the small telescope as with the large
telescope.