The second law of thermodynamics
General
Problem:
Give two statements of the second law of thermodynamics that are not
obviously identical in conceptual content but are in fact equivalent.
Solution:
- Concepts:
The second law of thermodynamics
- Reasoning:
We are supposed to express the second law of
thermodynamics in two not obviously identical ways.
- Details of the calculation:
(1) Heat cannot be taken in at a certain
temperature with no other change in the system and converted into work.
(2) The total entropy of a closed system is always increasing.
Problem:
Determine the work that can be obtained from the one cycle of the ideal
Carnot machine in which the working substance is a photon gas. The energy
density of this gas is given by the u = σT4 and the pressure is P =
(1/3)u.
Solution:
- Concepts:
The second law of thermodynamics
- Reasoning:
For an ideal, reversible engine Q1/T1 = Q2/T2.
- Details of the calculation:
For a Carnot engine we have
Q1/T1 = Q2/T2,
Wmax = (Qhigh - Qlow)reversible
= Qhigh - QhighTlow/Thigh = Qhigh(1
- Tlow/Thigh).
This is the same for all Carnot engines and does not depend on the working
fluid.
Problem:
What is the efficiency of a Carnot cycle operating between the same high (TH)
and low (TC) temperatures as the ideal gas in this circular cycle?
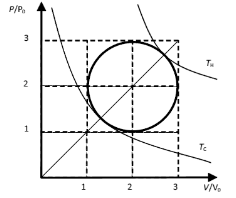
Solution:
- Concepts:
The Carnot cycle, the ideal gas law
- Reasoning:
The efficiency of a Carnot engine is (Thigh - Tlow)/Thigh.
We use the diagram (geometry) and the ideal gas law to find TH and TC.
- Details of the calculation:
For the circular cycle we have (P/P0 - 2)2 + (V/V0
- 2)2 = 1. (equation of a circle)
For TH and TC we have P/P0 = V/V0.
(straight line)
Therefore at those temperatures 2(P/P0 - 2)2 = 1, P/P0 = 2 + 2-½, for TH and P/P0 =
2 - 2-½, for TL.
Using the ideal gas law, PV = nRT we have
TH = [P0V0/(nR)][ 2 + 2-½]2
and TC = [P0V0/(nR)][ 2 - 2-½]2.
The efficiency of a Carnot cycle operating between TH and TC
is
eff = (TH - TC)/TH = [(2 + 2-½)2 - ( 2 - 2-½)2]/(2 + 2-½)2 =
77.19%
Heat engines
An ideal heat engine is powered by two reservoirs of equal heat capacity C,
which is temperature independent. As the engine works, the reservoirs gradually
equilibrate.
(a) Find the overall efficiency of the engine from the starting point where the
reservoirs are at temperatures T1 = 90 oC and T2
= 30 oC to the moment of complete equilibration.
(b) Now assume that a real heat engine is powered by the same reservoir with
initial temperatures T1 = 90 oC and T2 = 30
oC. Let C = 105 J/oC. The real engine's overall
efficiency is 20% of the overall efficiency of the ideal engine. Find the
change in entropy of the system once the reservoirs have equilibrated.
Problem:
A stretched rubber band contracts when heated under constant tension. Its
temperature increases when stretched adiabatically. The equation of state for
an idealized rubber band is J = αLT, where J is the tension in the rubber band,
L is its length, T is the absolute temperature and α is a constant.
For reversible processes we have for the rubber band TdS = dQrev = cLdT
- JdL.
The heat capacity of the band at constant length is cL = constant.
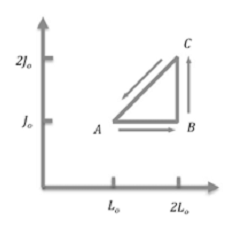
Consider a heat engine that uses a rubber band in the three-step cycle
shown.
Start with a stretched rubber band of length L0 , tension J0,
and temperature TA.
Take the band through a sequence of reversible processes.
A --> B:
The rubber band is stretched under constant tension J0 to a length 2L0
while in contact with a heat reservoir of temperature TB.
B --> C:
While in contact with a heat reservoir of temperature TC the tension
of the rubber band is increased from J0 to 2J0 at constant
length 2L0 .
C --> A:
While in contact with a heat reservoir of temperature TA, tension and
length decrease linearly from (2J0, 2L0) to (J0,
L0).
(a) Find the ratios TB/TA and TC/TA.
What is the ratio Thot/Tcold?
(b) Find the work done by the heat engine as it moves through one cycle A --> B
--> C --> A.
(c) During one cycle A --> B --> C --> A, how much heat is extracted from the
hot reservoir and how much heat is dumped into the cold reservoir?
(d) Find the efficiency of this rubber-band heat engine and compare it to the
efficiency of a Carnot engine operating between the same temperatures.
Solution:
- Concepts:
A simple heat engine, reversible processes
- Reasoning:
We are asked to take a simple heat engine through one cycle.
- Details of the calculation:
(a) A --> B:
JA = J0 = αL0TA, JB
= J0 = α2L0TB, TB = TA/2,
ΔT = TB - TA = -TA/2.
B --> C:
JB = J0 = α2L0TB, JC
= 2J0 = α2L0TC, TC = 2TB
= TA, ΔT = TC - TB = TA/2.
C --> A:
ΔT = 0.
Thot = TA, Tcold = TB, Tcold/Thot
= ½.
(b) A --> B:
ΔW = -J0L0 = -αL02TA.
B --> C:
ΔW = 0.
C --> A:
ΔW = -∫2L0L0JdL = -αTA∫2L0L0LdL
= (3/2)αL02TA.
ΔWtot = -αL02TA + (3/2)αL02TA
= ½αL02TA.
(c) A --> B:
ΔQ = -cLTA/2 - αL02TA.
B --> C:
ΔQ = cLTA/2.
C --> A:
ΔQ = +(3/2)αL02TA.
ΔQadded = (3/2)αL02TA + cLTA/2.
ΔQdumped = (cLTA/2 + αL02TA).
(d) Efficiency = ΔWtot/ΔQadded = 1 /(cL/(αL02
) + 3)
The efficiency of a Carnot engine operating between Thot = TA
and Tcold = TB is 1 - TB/TA = ½.
eff(Carnot)/eff(rubber band) = cL/(2 αL02 )
+ 3/2.
Problem:
A Carnot heat engine has the following entropy-temperature
diagram.
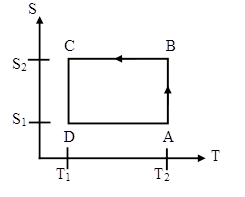
(a) Describe the cycle. For each segment identify the
process, say whether work is done by the working system or on it and whether
heat is added to the system or extracted from it.
(b) How much work is done by the system?
Solution:
- Concepts:
The Carnot cycle, isothermal and adiabatic processes,
entropy
- Reasoning:
The Carnot cycle consists of isothermal expansion, adiabatic expansion,
isothermal compression, and adiabatic compression.
- Details of the calculation:
(a) A --> B:
Isothermal expansion: The engine is in contact with a heat reservoir at
temperature T2. The engine does work.
ΔW = ∫PΔdV.
Heat is added to the working fluid.
B --> C: Adiabatic expansion: During
the expansion the temperature falls to T1.
The engine does work. ΔW =
∫PdV. No heat is added or extracted
from the working fluid.
C --> D:
Isothermal compression: The engine is in contact with a heat reservoir at
temperature T1. Work is done on the engine.
ΔW = -∫PdV.
Heat is extracted from the working fluid.
D --> A:
Adiabatic compression: During the compression the temperature rises to T1.
Work is done on the engine. ΔW = -∫PdV.
No heat is added or extracted from the working fluid.
(b) W = ΔQhigh -
ΔQlow
= ΔS*T2
- ΔS*T1 = (S2 - S1)*(T2
- T1).
Problem:
The operation of a gasoline engine is (roughly) similar to the Otto cycle.
A S-V diagram is shown.
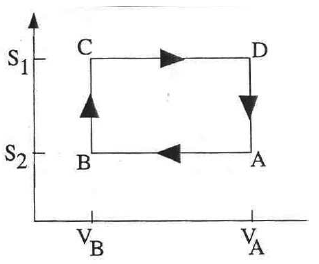
A → B: Gas compressed adiabatically.
B → C: Gas heated
isochorically (constant volume; corresponds to combustion of gasoline).
C →
D: Gas expanded adiabatically (power stroke).
D → A: Gas cooled
isochorically.
Compute the efficiency of the Otto cycle for an ideal gas
(with temperature-independent heat capacities) as a function of the compression
ratio VA/VB, and the heat capacity of the gas, CV
.
Solution:
- Concepts:
Heat engines
- Reasoning:
Efficiency = net work done by the engine divided by the
heat absorbed. We use the ideal gas law and the definition of the heat
capacity CV to find these quantities.
- Details of the calculation:
Energy conservation: dU = dQ - PdV, dS
= dQ/T, dU = TdS - PdV.
For an ideal gas we have PV = NkBT,
CVdT = dU.
Work done by the engine during one cycle: Wtot
= WA-->B + WC-->D = ∫A-->BPdV + ∫C-->DPdV.
Heat absorbed by the engine during one cycle: Qin = QB-->C
= ∫B-->CTdS.
Efficiency: eff = Wtot/Qin.
Finding Wtot:
WA-->B = ∫A-->BPdV =
-NkB∫VBVA (T/V)dV.
S = S2 =
constant along the path. We need to express T in terms of S and V.
dS =
dU/T + PdV/T = CVdT/T + NkBdV/V.
This integrates to
S = CV lnT + NkB lnV + constant.
Solving for T we
find: T = α*exp(S/CV)*V^(-NkB/CV), where α
is a constant.
WA-->B = -αNkBexp(S2/CV)∫VBVA
V^(-1 - NkB/CV))dV
= -α exp(S2/CV)
CV (VB^(-NkB/CV) - VA^(-NkB/CV)).
Similarly, WC-->D = α exp(S1/CV) CV
(VB^(-NkB/CV) - VA^(-NkB/CV)).
Wtot = α CV ((VB^(-NkB/CV)
- VA^(-NkB/CV))(exp(S1/CV)
- exp(S2/CV)).
Finding Qin:
Qin
= ∫S2S1 TdS = α*VB^(-NkB/CV)∫S2S1
exp(S/CV)dS
= α*CV*VB^(-NkB/CV)(exp(S1/CV)
- exp(S2/CV)).
eff = 1 - (VA/VB)^(NkB/CV).
For a monatomic ideal gas dU = (3/2)NkBdT = CVdT, NkB/CV
= 2/3, e = 1 - (VA/VB)-2/3.
Refrigerators, Air conditioners, Heat pumps
Problem:
A refrigerator uses 10 W of electrical power when it is
closed to keep the interior temperature stable. Use reasonable estimates for
any relevant temperatures to find an upper bound on the rate at which heat is
entering the refrigerator due to imperfect thermal insulation.
Solution:
- Concepts:
The second law of thermodynamics
- Reasoning:
For a reversible process Q1/T1 = Q2/T2.
This determines the best possible coefficient of performance of the
refrigerator.
- Details of the
calculation:
For a refrigerator we define the coefficient of performance COP as the ratio
of the amount of heat removed at the lower temperature to the work put into
the system.
COP = Qlow/W
= Qlow/(Qhigh - Qlow).
The best possible coefficient of performance is
COPmax = Qlow/(Qhigh
- Qlow)max
= Qlow/(Qlow(Thigh/Tlow) - Qlow)
= Tlow/(Thigh - Tlow).
Let us assume that Tlow = 4oC
= 277 K, and Thigh = 20oC. Then COPmax = 277/16.
So if the refrigerator were ideal, the heat
removed per second = 10W*277/16 = 173 W.
Upper bound on the rate at which heat is entering
the refrigerator: 173 W
Problem:
An electric freezer is turned on inside a tent for a long time. It is 0
oC outside the tent, +1 oC inside the tent, and -13 oC
inside the freezer. What would be the equilibrium temperature inside the tent
if another freezer is turned on inside the tent? The outside temperature
remains the same. The freezers are identical and follow the Carnot cycle.
Solution:
- Concepts:
Thermal equilibrium, the Carnot cycle
- Reasoning:
We have three regions:
Region 1: The inside of the refrigerator
Region 2: The inside of the tent.
Region 3: The outside reservoir at 273 K.
Assume region 1 and region 2 are in equilibrium with their surroundings.
Then the heat flowing into a region per unit time must equal the heat
flowing out per unit time.
- Details of the calculation:
Region 1: The heat flowing in is proportional to the temperature difference
T2 - T1.
Q1 = b(T2 - T1)
(ΔQ/Δt = -kAΔT/Δx , where k is the thermal conductivity, ΔT = (T2
- T1) is the difference in temperature between side 2 and side 1
of a layer of material of area A, Δx is the thickness of that layer, and ΔQ/Δt
is the amount of heat that flows from side 1 to side 2 through the layer of
material per unit time. )
This heat Q1 is removed again by the refrigerator.
Region 2: The heat flowing in is the heat dumped by the refrigerator.
Q2 = Q1T2/T1 = b(T2 -
T1)T2/T1
(For the Carnot cycle Q1/T1 = Q1/T2.)
The heat flowing out is Q3 flowing into region 3 and Q1 flowing into region
1. Q3 is proportional to the temperature difference T2 - T3.
Q3 + Q1 = a(T2 - T3) + b(T2
- T1)
In equilibrium Q2 = Q3 + Q1.
a(T2 - T3) + b(T2 - T1) = b(T2
- T1)T2/T1.
We have a/b = (T2 - T1)(T2/T1 -
1)
With the data for the single refrigerator we can evaluate a/b.
T3 = 273 K, T2 = 274 K, T1 = 260 K, a/b =
0.7538.
For the two refrigerators the wall area is doubled, so b --> b' = 2b, a/b' =
0.3769
T3 and T1 do not change we have to solve a/b' = (T2
- T1)(T2/T1 - 1) for T2.
T2 = 275.43 K = 2.43 oC.
Problem:
A nursery uses natural gas heating to keep the greenhouses at 30 oC
all year. An engineer points out that the water at the bottom of a nearby lake
is at a constant temperature of 5 oC, and that he can build an ideal
heat pump that will work at maximum possible efficiency to pump heat from this
lake water into the greenhouses. He claims that the nursery will come out ahead
with his system, even though it uses electricity instead of natural gas at three
times the cost per Joule. Is he right? Neglecting capital and maintenance
costs by what factor would their energy bill change?
Solution:
- Concepts:
The coefficient of performance for a heat pump
- Reasoning:
The coefficient of performance for a heat pump is the ratio of the energy
delivered at the higher temperature to the work put into the system, COP = Qhigh/(Qhigh
- Qlow). Knowing the COP, we can determine the cost of the
delivered energy.
- Details of the calculation:
The best possible coefficient of performance is
COPmax(heat pump) = (Qhigh/(Qhigh - Qlow))max
= Thigh/(Thigh - Tlow) = Troom/(Troom
- Toutside)
Here COPmax = 303/25.
The gas heating system and the heat pump have to deliver the same amount of
energy.
For the heat pump: energy paid for = 25/303 times energy delivered
For the gas heater: energy paid for = energy delivered
Cost for heat pump delivery = 3*25/303 times cost for gas heater delivery
Their new bill would be approximately ¼ their old bill.




