Boltzmann statistics
Problem:
Neutrons are released into an evacuated cubical chamber and lowered to a very
cold T = 1 mK temperature. What is their mean height above the floor of
the chamber, once their gravitational energy has reached an equilibrium
distribution? (Show work! Derive a formula and quote a number.)
Solution:
- Concepts:
Boltzmann distribution
- Reasoning:
We have a macroscopic-size chamber.
- Details of the calculation:
The probability per unit length of
finding the neutron at height z is
P(z) = P(0)exp(-mgz/(kBT)).
1 = P(0)∫0∞exp(-mgz/(kBT))dz = P(0)kBT/(gm),
P(0) = gm/(kBT).
<z> = gm/(kBT) ∫0∞z
exp(-mgz/(kBT)) dz = kBT/(gm).
kB =
1.380658 *10-23 J/K.
mn = 1.6749286 * 10-27
kg.
<z> = 0.841 m.
Problem:
Consider ideal gas particles
of mass m at temperature T. What is the average speed <v> (not the rms <v2>½
) of the particle in terms of m, T and the Boltzmann constant kB?
∫0∞x2n exp(-x2)dx = π½
(1*3*5* ... *(2n -1))/2n+1
Solution:
- Concepts:
Boltzmann statistics
- Reasoning:
The Boltzmann distribution gives the probability of
finding particles with energy E at a given temperature T.
- Details of the calculation:
Boltzmann statistics: Ni/N =
giexp(-Ei/kT)/∑i(giexp(-Ei/kT))
Here gi is the degeneracy.
For this problem:
n(v)dv/N =
P(v)dv = g(v)exp(-mv2/(2kT))dv/∫0∞g(v')exp(-mv'2/(2kT))dv'
= v2exp(-mv2/(2kT))dv/∫0∞v'2exp(-mv'2/(2kT))dv',
since g(v) is proportional to v2.
1/∫0∞v'2exp(-mv'2/(2kT))dv'
= (m/(2kT))3/2(4/π½).
So <v> = ∫0∞
vP(v)dv = (m/(2kT))3/2(4/π½) ∫0∞v3exp(-mv2/(2kT))dv
= (m/(2kT))3/2(2/π½) ∫0∞v2exp(-mv2/(2kT))dv2
= (8kT/(mπ))½.
Some additional remarks:
The Boltzmann
distribution describes the distribution of energy among classical
(distinguishable) particles.
P(E) = A exp(-E/(kT))
It can be used to
evaluate the average energy per particle in the circumstance where there is
no energy-dependent density of states to skew the distribution.
To
represent the probability for a given energy, it must be normalized to a
probability of 1.
∫0∞ Aexp(-E/(kT))dE = 1 ==> A =
1/(kT).
This normalized distribution function can then be used to
evaluate the average energy.
<E> = (kT)-1∫0∞
Eexp(-E/(kT))dE = kT.
This is the average energy if the energy is
randomly distributed among the available energy states and there is no
energy-dependent density of states.
Note that this average energy for
randomly distributed energy is not the same as the average kinetic energy.
The average kinetic energy is found by evaluating
<v2> = ∫0∞
v2P(v)dv = (m/(2kT))3/2(4/π½)
∫0∞v4exp(-mv2/(2kT))dv = 3kT/m.
½m<v2> = (3/2)kT.
We have an energy dependent density of
states.
We can use E instead of v as the variable we integrate over.
g(v)dv = g(E)dE = g(E)mvdv, g(E) = g(v)/(mv).
Since g(v) is proportional
to v2, g(E) is proportional to E½.
We can then
rewrite all the integrals in terms of E instead of v.
Problem:
Suppose a small meteorite makes a hole of area A = 1 mm2 in the wall
of a spaceship. The habitable volume of the spaceship is V = 10 m3.
The temperature of the air in the spaceship is
T = 27o C and the pressure P = 105 kPa. The molar mass of air is M =
29 g /mole. Estimate, how much time will be available to astronauts to put on
spacesuit, if the pressure should not drop by more than to 50% of its initial
value.
Maxwell-Boltzmann speed distribution: f(v) = (m/(2πkT))3/2 4πv2exp(-mv2/(2kT)
Solution:
- Concepts:
The Maxwell Boltzmann distribution, ideal gas
law
- Reasoning:
We need to find the escape rate Resc = -dN/dt,
then we can integrate to find N(t). The rate with which the molecules escape from the hole
depends on their average speed.
- Details of the calculation:
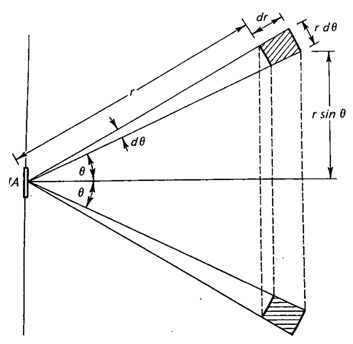
Consider an area dA and gas molecules with speeds between v and v + dv in a ring at a distance between r and r + dr from dA. Assume that the gas molecules travel in straight lines.
The probability that gas molecules from the ring will reach the area dA is
dA cosθ/(4πr2). This is the solid angle subtended by dA at any dV in
the ring.
The number of molecules from the ring reaching dA with speeds between v and v +
dv is
nf(v)dv2πr2sinθ dr dθ dA cosθ/(4πr2) = nf(v)dv sinθ dr dθ
dA cosθ/2.
Here n = N/V is the density of the gas.
The total number of molecules with speeds between v and v + dv reaching dA in a
time interval dt comes from rings with radii between r = 0 and dr = vdt.
It is
therefore given by
∫0π/2 dθ nf(v)dv dA vdt sinθ cosθ/2 = ¼ n f(v) dv dA
vdt.
Integrating over all speeds we find Resc = ¼ n dA <v>,
where Resc
denotes rate at which gas molecules reach dA and <v> = ∫0∞
vf(v) dv.
For the Maxwell Boltzmann speed distribution we find
<v> = (8kT/(mπ))½, where m is the mass of a gas molecule.
Resc = (P/kT) dA (kT/(2mπ))½.Resc = -dN/dt is the rate at which molecules are
escaping from the
chamber.
dN/dt = -(N/V) dA (kT/(2mπ))½.
N(t) = N0exp(-Ct), where C = (dA/V) (kT/(2mπ))½ = (dA/V)
(RT/(2Mπ))½
If N/N0 = ½, then t = ln(2)/C.
C = (10-6 m2/10 m3)*[(8.314 J/K mole * 300
K)/(2* 29*10-3 kg/mole * π)]½ = 1.71*10-3/s.
t = 592 s ~ 10 minutes.
Quantized energies
